Dr Westenfeld is head of the intensive care medicine and heart failure section at University Hospital, Dusseldorf, Germany. His research interests include the pathomechanisms of cardiovascular calcification, interventional treatment strategies in high-risk patients, and myocarditis and transplant rejection.
Dr Westenfeld began by describing the current state of understanding of pulmonary congestion in cardiogenic shock (CS). The evolution of systemic inflammatory response and multiple organ dysfunction syndrome following cardiopulmonary resuscitation may affect postcardiac- arrest-syndrome.1 Acute lung injury is an unrecognised problem in patients on extracorporeal life support (ECLS) who undergo implantation of a long-term mechanical circulatory support (MCS) device.2 In addition, early progression of pulmonary oedema (within 24 hours) has been found to predict mortality in patients with extracorporeal membrane oxygenation (ECMO).3 Increased pulmonary congestion in patients with ECMO carries the same mortality risk as dialysis. However, there have been no studies on the evolution of pulmonary congestion in CS.
Dr Westenfeld’s team proposed the hypothesis that that pulmonary congestion in CS develops differently according to the type of MCS (Impella versus intra-aortic balloon pump [IABP]), and also that early increase of pulmonary congestion in CS is associated with adverse outcome and recovery. In order to investigate this hypothesis, a method of quantifying pulmonary congestion without the use of a CT scan was required. The Halperin score identifies six areas from chest X-rays and assigns scores according to the observed opacification. Congestion is then classified according to the Halperin score as mild (score 100), moderate (230) or severe (310).
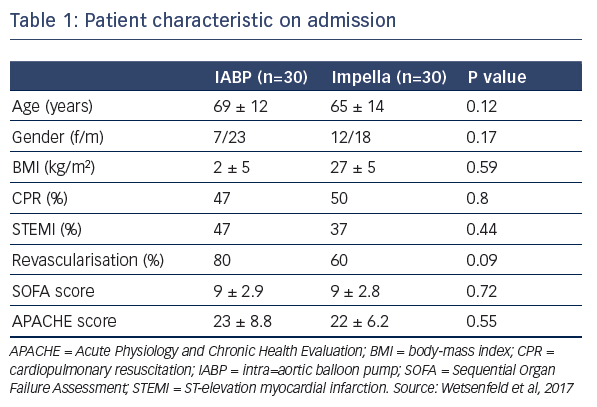
Dr Wentenfeld presented a retrospective study that identified 74 patients with CS who had received either IABP (January 2012– May 2015, n=43) or Impella (April 2014–June 2016, n=31) support.4 After excluding patients who died during the blanking period or those who required ECLS, 60 patients remained for analysis; 30 who received IABP and 30 who received the Impella. On admission, patient characteristics were similar between groups (see Table 1). They had high serum lactate during MCS support, which was higher in the Impella group (see Table 1). Similarly, troponin levels, inotropic score, and levels of lactate dehydrogenase were also high, but not significantly different between groups. Data suggest that the patients treated with Impella may have been associated with increased tissue perfusion, which could lead to the observed higher lactate levels, or this group may have included sicker patients, supported by the data that an upgrade to ECLS or left ventricular assist device was needed in 10 % of the IABP group versus 33 % in the Impella group (P=0.03). Pneumonia was reported in 76 % of patients receiving IABP and 56 % receiving the Impella (P=0.18). Hospitalisation in the intensive care unit was required for 15±15 days and 24±14 days in the IABP and Impella groups, respectively (P=0.03). Total hospitalisation was 30±35 days and 48±30 days, respectively (P=0.03). At 30 days, survival was 42 % and 48 %, respectively (P=0.8).
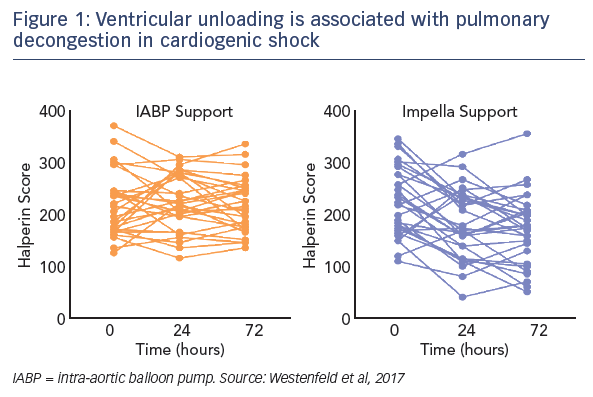
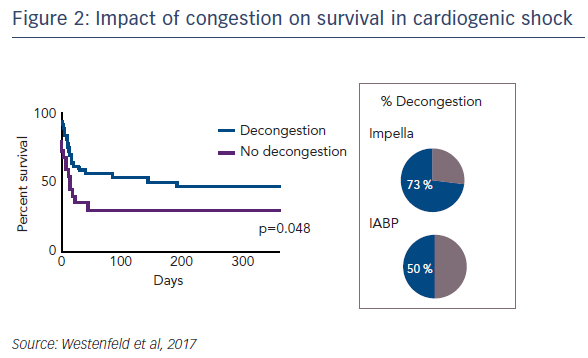
Regarding the impact of ventricular unloading by the Impella on pulmonary congestion, a significant decrease in the Halperin score at 72 hours was observed in patients treated with Impella compared with those treated with IABP (see Figure 1). When the entire cohort was divided into patients who did or did not experience pulmonary decongestion as defined by the Halperin score, an association was seen between reduction of pulmonary congestion within the first 24 hours and improved survival in CS (see Figure 2). However, this Impella-dependent effect did not translate directly into a significant survival benefit in the overall cohort. Dr Westenfeld commented that in the cohort he examined, decongestion was achieved in 73 % of Impella-supported patients, but only 50 % of IABP patients.
In conclusion, in this single centre study, data suggest that early progression of pulmonary oedema is associated with poor outcome in CS. Left ventricular unloading by Impella may more effectively facilitate pulmonary decongestion in CS compared with IABP support. This study was limited by its small sample size and retrospective design. There is a need for a large-scale analysis of outcomes and confounders in large registry studies. In addition, prospective analysis is needed of pulmonary congestion in CS, using early assessment by ultrasound, guiding escalation strategies, and investigating weaning and haemodynamics. Finally, mechanistic studies in large animal models will help elucidate the effects at the molecular level in terms of mitochondrial function, reactive oxygen species production and the effects of unloading on inflammation.