Historical Perspective and Early Experience
Historically, coronary artery bypass grafting (CABG) surgery has been the gold standard treatment for left main coronary artery (LMCA) disease. However, with the developments and advancements in the interventional cardiology field, percutaneous coronary intervention (PCI) has become a safe alternative management option to CABG in select patients. Today, LMCA disease is identified in about 5% of patients undergoing primary coronary intervention for evaluation of ischaemia.1
The first left main PCI was performed by Andreas Gruntzig in 1978 using plain old balloon angioplasty (POBA). In his initial experience, Gruntzig performed POBA in 50 patients over a period of 18 months; POBA was deemed successful in 32 patients. Using POBA, mean left main stenosis improved from 84% to 34% (p<0.001) with coronary pressure gradient improving from a mean of 58 mmHg to 19 mmHg (p<0.001).2 POBA alone was associated with acute and subacute vessel closure, elastic recoil, late vascular remodelling and re-narrowing of coronary arteries. This led to the development of coronary stents and their progressive refinement from bare-metal stents (BMS) to drug-eluting stents (DES). These newer stents addressed the shortcomings of BMS, specifically in-stent restenosis. Furthermore, the development of novel anti-platelet medications has led to significant reductions in the incidence of major adverse cardiac events (MACE).
Important Randomised Clinical Trials
Early Evidence
In a 2011 study, Boudriot et al. compared PCI using sirolimus-eluting stents with CABG for the management of LMCA disease.3 Overall 201 patients were randomised to receive PCI (n=100) or CABG (n=101), with follow-up for 12 months. Mortality and MI rates were comparable, but PCI was associated with higher rates of repeat revascularisation and CABG was associated with higher rates of stroke. The combined primary endpoint was noninferiority in freedom from major adverse cardiac events and the need for target vessel revascularisation within 12 months; this was observed in 13.9% of patients after CABG, as opposed to 19.0% after PCI (p=0.19 for noninferiority).
Combined rates for death and MI were comparable (7.9% in the CABG group versus 5.0% in the PCI group; noninferiority p<0.001), but PCI was inferior to CABG for repeat revascularisation (5.9% versus 14.0%; noninferiority p=0.35). Perioperative complications (including two patients having strokes) were higher after CABG (4% in PCI versus 30% in CABG; p<0.001). Freedom from angina was similar between the groups (p=0.33).3
SYNTAX
In 2014, subgroup analysis of the Synergy Between PCI With TAXUS And Cardiac Surgery (SYNTAX) trial compared left main PCI using first-generation DES with CABG.4 A total of 357 patients received PCI and 348 patients received CABG. At 5 years, in patients with SYNTAX score <33 (a scoring system that quantifies angiographic lesion complexity) the incidence of MACE was similar in the PCI and CABG groups. However, there was a higher incidence of target vessel revascularisation (TVR) in the PCI group than in the CABG group.
In patients with a high SYNTAX score (≥33), CABG had favourable outcomes compared with PCI. Major adverse cardiac and cerebrovascular events (MACCE) rates at 5 years were 36.9% in the PCI arm and 31.0% in the CABG arm (HR 1.23; 95% CI [0.95–1.59]; p=0.12). The mortality rate was 12.8% and 14.6% in the PCI and CABG arms, respectively (HR 0.88; 95% CI [0.58–1.32]; p=0.53). Stroke rate was significantly higher in the CABG arm (PCI 1.5% versus CABG 4.3%; HR 0.33; 95% CI [0.12–0.92]; p=0.03) while repeat revascularisation was significantly higher in the PCI arm (26.7% versus 15.5%; HR 1.82; 95% CI [1.28–2.57]; p<0.01). MACCE was similar between the two arms in patients with low/intermediate SYNTAX scores but significantly higher in PCI patients with SYNTAX scores ≥33.
PRECOMBAT
The Bypass Surgery Versus Angioplasty Using Sirolimus-Eluting Stent in Patients With Left Main Coronary Artery Disease (PRECOMBAT) trial was published in 2015.5 This multicentre trial in South Korea randomised 300 patients to PCI and 300 patients to CABG. There was no significant difference in rates of MACE in patients undergoing PCI with sirolimus-eluting stents compared with patients undergoing CABG over 5 years of follow-up. There was a higher incidence of revascularisation in the PCI group compared with the CABG group.
At 5 years, MACCE occurred in 52 patients in the PCI group and 42 patients in the CABG group (cumulative event rates of 17.5% and 14.3%, respectively; HR 1.27; 95% CI [0.84–1.90]; p=0.26). The two groups did not differ significantly in terms of death from any cause, MI, or stroke as well as their composite (8.4% and 9.6%; HR 0.89; 95% CI [0.52–1.52]; p=0.66). Ischaemia-driven TVR occurred more frequently in the PCI group than in the CABG group (11.4% and 5.5%, respectively; HR 2.11; 95% CI [1.16–3.84]; p=0.012).5
LE MANS
The multicentre Left Main Coronary Artery Stenting (LE MANS) trial randomised 105 patients to PCI (n=52) or CABG (n=53). Of those undergoing PCI, only 35% received DES; the majority received BMS. The study showed a favourable but statistically non-significant outcome in favour of PCI compared to CABG over a 10-year follow-up period. At 10 years, there was a trend towards higher ejection fraction with PCI compared with CABG (54.9% ± 8.3 versus 49.8% ± 10.3; p=0.07), lower mortality (21.6% versus 30.2%; p=0.41) and lower MACCE (51.1% versus 64.4%; p=0.28). Although there were no statistically significant differences between groups, numerically the findings were favour of stenting. Similarly, there was no difference in the occurrence of MI (8.7 versus 10.4%; p=0.62), stroke (4.3 versus 6.3%; p=0.68), and repeat revascularisation rates (26.1% versus 31.3%; p=0.64). The probability of very long-term survival up to 14 years was comparable between PCI and CABG (74.2% versus 67.5%; p=0.34; HR 1.45 95% CI [0.67–3.13]). However, there was a trend towards higher MACCE-free survival in the PCI group (34.7% versus 22.1%; p=0.06; HR 1.71 95% CI [0.97–2.99]).6
EXCEL and NOBLE
More recently the Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) and Nordic-Baltic-British Left Main Revascularization Study (NOBLE) trials were published.7,8 These two multicentre randomised clinical trials were performed to assess the outcomes of second-generation DES in left main PCIs compared with CABG.
In the EXCEL trial, a total of 1,905 patients with low–intermediate SYNTAX score of <33 were randomised to PCI versus CABG.7 The primary endpoint was defined as a composite of all-cause death, MI and stroke at 3 years. The primary endpoint occurred in 15.4% of the patients in the PCI group and in 14.7% of the patients in the CABG group (difference of 0.7 percentage points; upper 97.5% confidence limit 4.0 percentage points; p=0.02 for noninferiority; HR 1.00; 95% CI [0.79–1.26]; p=0.98 for superiority).
The secondary endpoint was defined as a composite endpoint of all-cause death, MI or stroke at 30 days. It occurred in 4.9% of the patients in the PCI group and in 7.9% in the CABG group (p<0.001 for noninferiority, p=0.008 for superiority). The secondary endpoint event of death, stroke, MI, or ischaemia-driven revascularisation at 3 years occurred in 23.1% of the patients in the PCI group and in 19.1% in the CABG group (p=0.01 for noninferiority, p=0.10 for superiority). In summary, 30-day MACE was lower in PCI group, but similar for both the PCI and CABG at 3-year follow-up.
In the NOBLE trial, a total of 1,201 patients with low–intermediate SYNTAX score were randomised to PCI using biolimus-eluting stents versus CABG.8 The 30-day outcomes were similar to those of the EXCEL trial, but the 5-year outcomes MACCE for PCI were inferior to CABG, mainly due to repeat revascularisation, an endpoint not included in the composite endpoint of EXCEL but in keeping with most of the cumulative literature on this subject in prior randomised trials. At 5 years, estimates of MACCE were 29% for PCI (121 events) and 19% for CABG (81 events), HR 1.48 (95% CI [1.11–1.96]), exceeding the limit for non-inferiority. CABG was significantly better than PCI (p=0.0066). The as-treated estimates were 28% versus 19% (HR 1.55; 95% CI [1.18–2.04]; p=0.0015). Comparing PCI with CABG, 5-year estimates were 12% versus 9% (HR 1.07; 95% CI [0.67–1.72]; p=0.77) for all-cause mortality, 7% versus 2% (HR 2.88; 95% CI [1.40–5.90]; p=0.0040) for non-procedural MI, 16% versus 10% (HR 1.50; 95% CI [1.04–2.17]; p=0.032) for any revascularisation, and 5% versus 2% (HR 2.25; 95% CI [0.93–5.48]; p=0.073) for stroke.
It is important to note that the primary endpoint in EXCEL trial differed from all other major left main trials including NOBLE because it excluded repeat revascularisation as a primary endpoint and added periprocedural MI within the composite endpoint. Furthermore, the NOBLE investigators excluded periprocedural MIs, which were captured by the EXCEL investigators. It is notable that although the primary DES used in NOBLE was the biolimus stent, 10% of patients received a first-generation DES before the switch to biolimus was made. On the other hand, EXCEL exclusively used the second-generation evirolimus stent (Xience).
MAIN-COMPARE Registry
In 2018, in the observational cohort study of the Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty versus Surgical Revascularization (MAIN-COMPARE) registry, the 10-year outcomes of PCI and CABG were assessed.9 The overall cohort found that there was no significant difference in the adjusted risk of death and composite outcomes between both groups at 10 years. However, there was a higher rate of TVR in the PCI group.
Meta-analyses
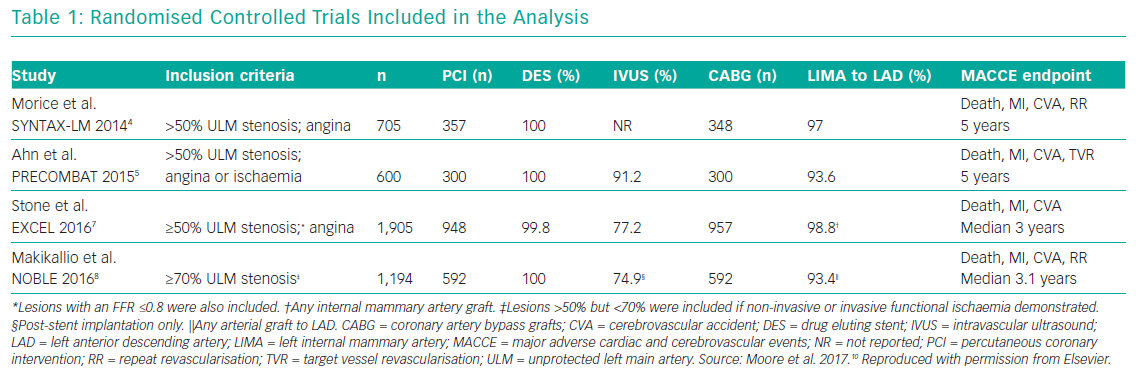
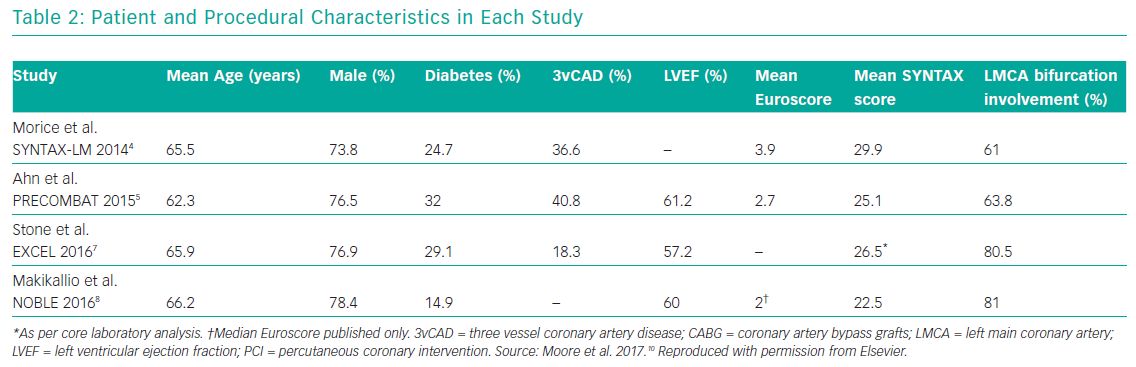
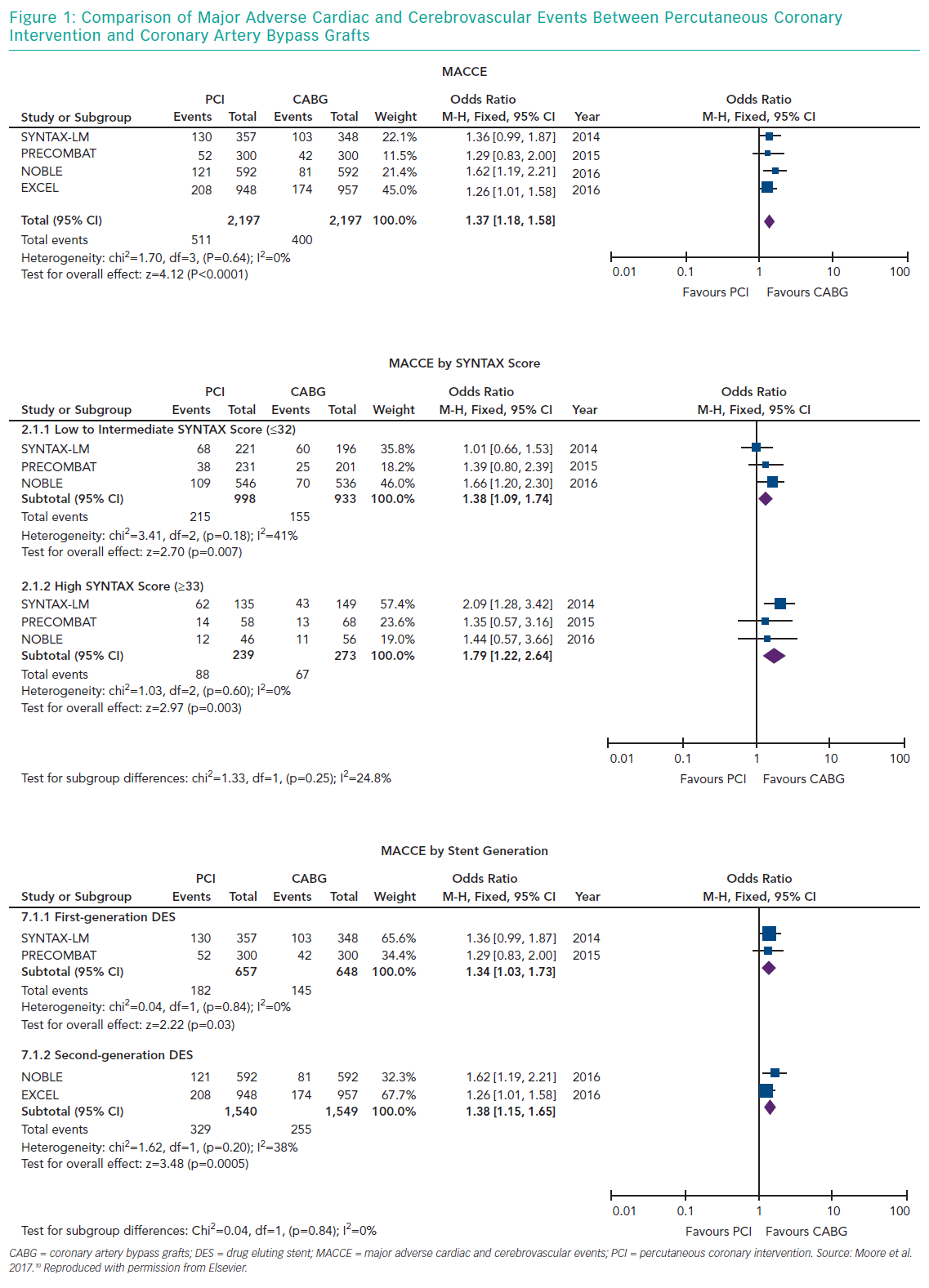
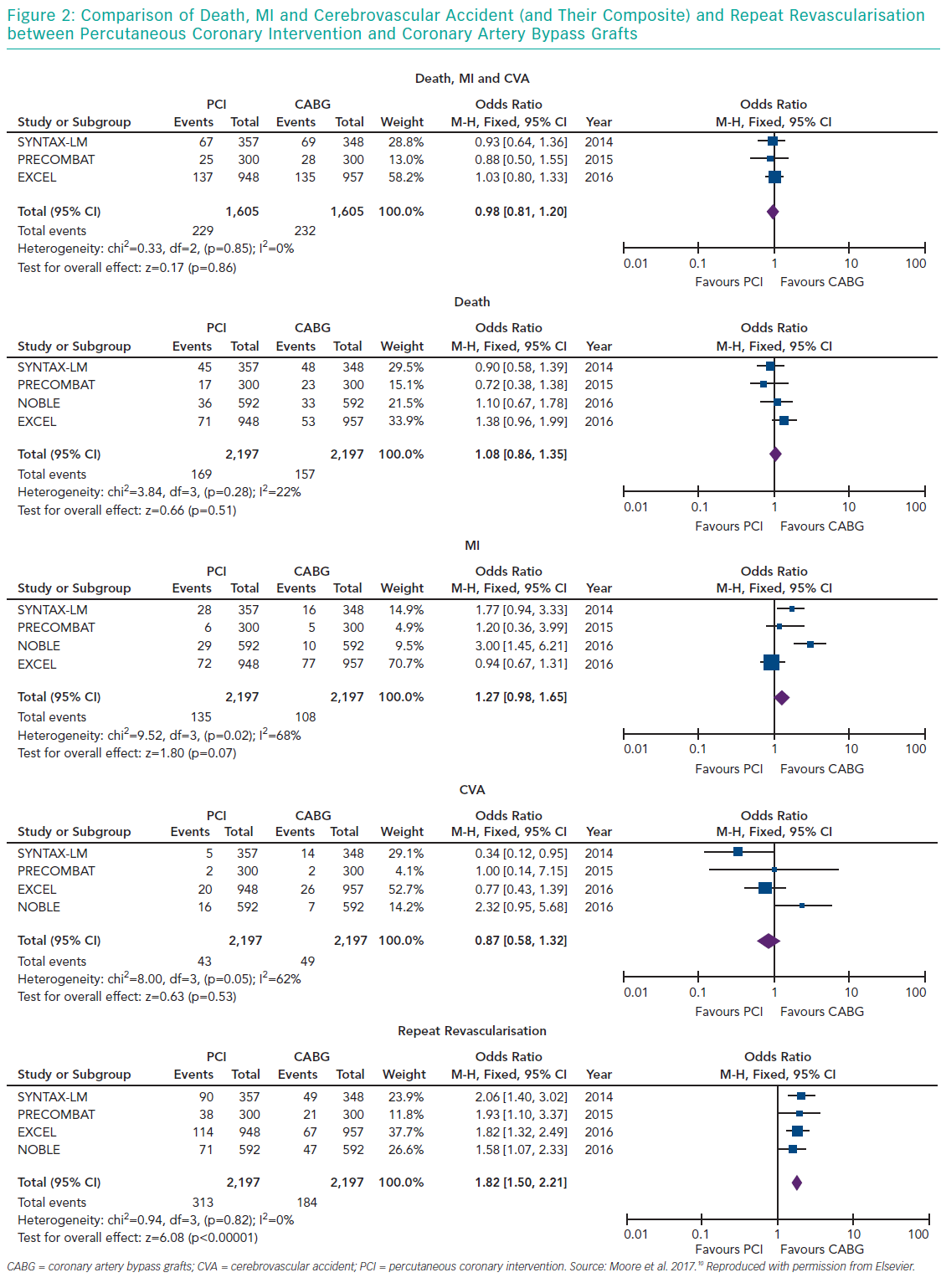
Several meta-analyses of these major clinical trials have shown that PCI has similar rates of MACE compared to CABG over 3–5 years in the management of LMCA disease, with PCI being associated with higher repeat revascularisation rates and CABG being associated with higher stroke rates in the early follow-up period. Moreover, these meta-analyses also showed that the use of second-generation DES was associated with lower revascularisation rates compared to first generation DES. Moore et al. analysed four major clinical trials in their meta-analysis (SYNTAX, PRECOMBAT, EXCEL and NOBLE; Tables 1 and 2). 10 The incidence of MACCE at 3 to 5 years of follow-up was significantly higher with PCI compared to CABG (23.3% versus 18.2%, OR 1.37; 95% CI [1.18–1.58]; p=<0.0001; I2=0%) and was largely driven by more repeat revascularisation procedures among patients treated with PCI. There was no statistically significant difference in rates of mortality, MI or stroke (either individually or when these outcomes were combined as a composite endpoint). Figures 1 and 2 summarise MACCE with an analysis by SYNTAX score and generation of stent.10–12
The CathPCI Registry
More recently in 2019, Valle et al. reviewed data from the National Cardiovascular Data Registry (NCDR) CathPCI registry reflecting current left main PCI outcomes in a real-world setting. The investigators compared outcomes collected in the NCDR registry with participants of the EXCEL and NOBEL trials. Patients from the CathPCI registry were significantly older, had more medical comorbidities, more frequently suffered stroke, and intravascular imaging was used less frequently.13
Role of Intravascular Imaging in Left Main Coronary PCI
Assessing the severity of stenosis of the LMCA with angiography has challenges. These include a short left main, obscured lesions from overlapping vessels or from reflux of contrast around the catheter in the aortic sinus, overestimation of the severity of the lesion particularly in the left anterior oblique projection with cranial angulation, catheter induced spasm, ambiguous eccentric lesions, underestimation of stenosis due to diffuse coronary artery disease and calcification.14 Hence, accurate assessment frequently requires additional evaluation to determine the appropriate revascularisation strategy. For high-quality cross-sectional imaging, intravascular ultrasound is particularly useful for assessment of both lumen and wall characteristics, positive remodelling and plaque burden.15 Compared to infrared light of optical coherence tomography (OCT), intravascular ultrasound has a higher tissue penetration and does not have the limitations of OCT in imaging the left main ostium.16 The minimal luminal area (MLA) is the most accepted variable for detecting a significant lesion, with a sensitivity of 93% and of specificity 95%, with MLA <6.0 mm2. Yet this was determined in predominantly western populations. MLA cut-off value can vary within different populations due to difference in body mass and vessel size. In asian patients, MLA <4.8 mm2 and MLA <4.1 mm2 were found to be significant for left main disease and had a sensitivity of 77%, 95% and specificity 82%, 83% respectively.17–19
Another modality to assist in the decision making process is physiological assessment of the lesion using Fractional flow reserve (FFR) with a cut-off of <0.08 indicating an important flow-limiting stenosis. A limitation to FFR is its inability to accurately determine the significance of the stenosis in the presence of tandem lesions. However, FFR may still play a significant role in the treatment of left main coronary artery bifurcation disease. For example, it can be useful in determining the significance of an ostial stenosis of the side branch (usually the left circumflex artery) and the need for a two-stent strategy.20–21
European and American Guidelines
The standard of care for LMCA disease for many years has been CABG over medical therapy because of mortality benefit.22
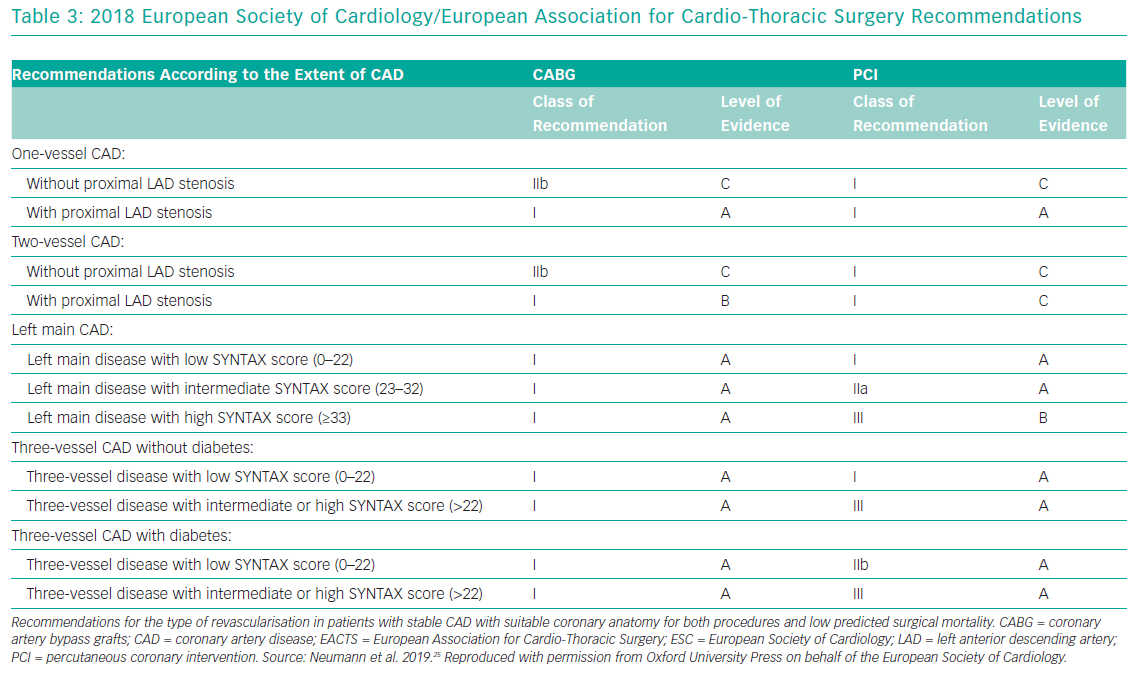
Over the past decade the treatment of LMCA disease has been the subject of multiple randomised controlled trials that have compared PCI to the gold standard of CABG. Based on results of these trials, the European Society of Cardiology (ESC) and the American College of Cardiology (ACC)/American Heart Association (AHA) have produced recommendations (Table 3).
In the 2014 ESC guidelines, patients with unprotected LMCA disease, stenosis of more than 90% with stable angina or silent ischaemia, or stenosis >50%–90% with documented ischaemia or FFR <0.80, should undergo revascularisation (class I, level of evidence A).23 With low surgical mortality and suitable coronary anatomy, CABG is recommended (Class I, level of evidence B). PCI is also recommended, depending on the SYNTAX score: <22 (class I, level of evidence B); 23–32 (class IIa, level of evidence B); and >32 (class III, level of evidence B).
The ACC/AHA 2014 guidelines indicate that PCI is a reasonable alternative to CABG for LMCA disease in patients with, ostial or trunk LMCA disease and a low SYNTAX score with an increased surgical adverse outcomes Society of Thoracic Surgery predicted mortality 5% (class IIa, level of evidence B).24 PCI is also reasonable in patients with unstable angina/non-ST-elevation MI when the LMCA disease is the culprit lesion and the patient is not a surgical candidate (class IIa, level of evidence B). When a patient presents with ST-elevation-MI and the LMCA disease is the culprit lesion with a distal coronary flow is less than thrombolysis in MI grade 3. PCI can be performed more rapidly and safely than CABG (class IIa, level of evidence C). PCI can be an alternative to CABG in select patients for LMCA disease with a low to intermediate risk of PCI procedural complications and an intermediate to high likelihood of good long-term outcomes (class IIb, level of evidence B). These guidelines are based on the SYNTAX trial and other smaller randomised trials that are underpowered, including the LE MANS trial and PRECOMBAT. Results from the EXCEL and NOBLE trials have not yet been incorporated into the guidelines.
Conclusion
In conclusion, left main PCI can be performed safely in select patients. The decision to proceed with PCI versus CABG is best made through a multidisciplinary approach consisting of a clinical cardiologist, interventional cardiologist and a cardiac surgeon. It entails consideration of the patient’s preferences and expectations, comorbidities, the estimated surgical risk, the complexity of coronary anatomy and the patient’s ability to comply with dual antiplatelet therapy. Appropriate evaluation of the lesion by coronary angiography and intravascular imaging coupled with operator expertise remains paramount to the decision-making process and strategy. Furthermore, all patients with LMCA disease will require optimal guideline-directed secondary prevention and lifestyle intervention in addition to the chosen revascularisation strategy.