Valvular heart disease (VHD) is encountered in approximately 1% of pregnancies, significantly increasing both maternal and foetal risk.1,2 Rheumatic VHD remains the most common form in non-Western countries, whereas congenital heart disease dominates in the Western world.3,4 As increasing numbers of women with congenital heart disease are reaching childbearing age, the prevalence of women of childbearing age with significant cardiac pathology is also increasing.5 Other causes of VHD in younger women include myxomatous mitral valvular disease (mitral valve prolapse), prior endocarditis, valvular disease associated with systemic disorders (Marfan’s syndrome, systemic lupus erythematosus, inflammatory vascular disorders) and radiation-induced valvular disease.6
Pregnant women with VHD are at risk of cardiac decompensation due to the haemodynamic changes that occur during pregnancy, including increases in heart rate, stroke volume and cardiac output (CO).3 The risk of complications varies according to the type and severity of the underlying VHD. However, stenotic valve lesions are generally less well tolerated during pregnancy than regurgitant lesions, because increased CO increases the transvalvular gradient by approximately 50%, mainly between the first and second trimesters, worsening the prognosis of the patient and the foetus.7,8 Moreover, pregnancy is a hypercoagulable state associated with an increased risk of thromboembolism.9
The management of VHD is particularly challenging in pregnant women because both the maternal and foetal prognoses are at stake, and need to be taken into account.10 In this article, we review the main VHDs that are encountered during pregnancy, and suggest management strategies based on the 2018 European Society of Cardiology (ESC) recommendations for the management of pregnant women with VHD.11 We also provide an overview of classical and new transcatheter structural therapeutic options, with a special focus on radiation exposure and anticoagulation drug management.
Prevention and Risk Assessment
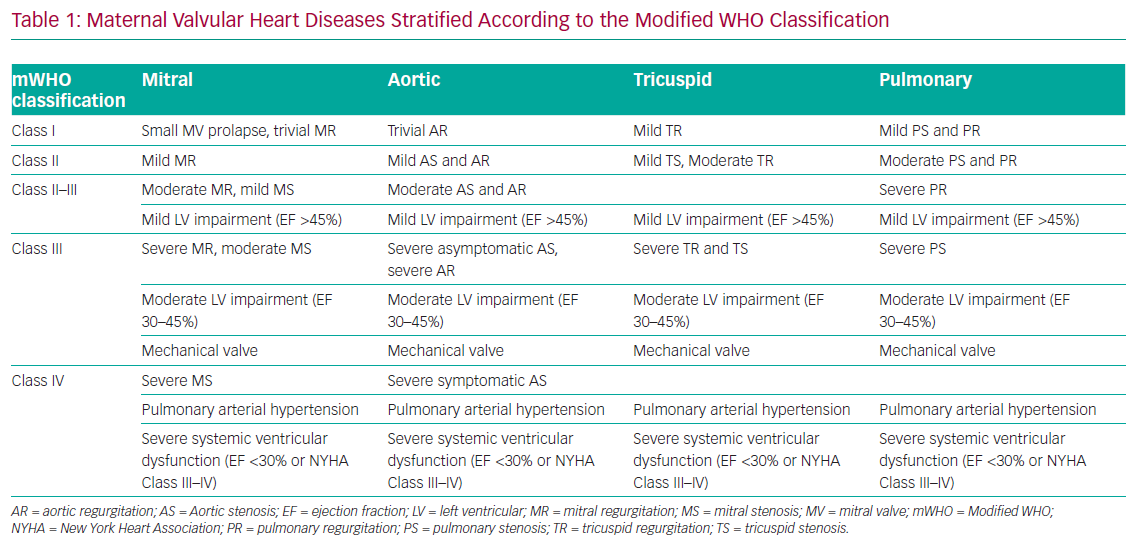
European guidelines recommend performing a risk assessment in all women with known cardiac diseases of childbearing age before conception using the modified WHO (mWHO) classification of maternal cardiovascular risk in order to plan the appropriate management (Table 1).11 VHD in pregnant women is heterogeneous and its management varies from simple surveillance (mWHO Class I) to contraindication or termination of pregnancy (mWHO Class IV).11
The current ESC guidelines introduced the notion of the pregnancy heart team. Any women with a moderate or high risk of complications during pregnancy (mWHO Class II–III and above) should be referred to for prepregnancy counselling and management during pregnancy and around delivery in order to anticipate and potentially avoid complications.11 The pregnancy heart team is a multidisciplinary team composed of at least a cardiologist, an obstetrician and an anaesthesiologist, all with expertise in the management of high-risk pregnancies in women with heart disease in an expert centre. Other additional experts may be involved where appropriate, such as geneticists, cardiothoracic surgeons, paediatric cardiologists, pneumologists, foetal medicine specialists, neonatologists, haematologists and nurse specialists.
All patients with high-risk VHD (mWHO Class IV) should be counselled against pregnancy or considered for prepregnancy interventions, particularly if the valve lesion is amenable to percutaneous intervention or surgical repair.11
Indications for Cardiac Interventions
Indications for intervention (surgical or transcatheter) do not differ between women who contemplate pregnancy and other patients. The only exception to this rule in the field of VHD is women with at least moderate mitral stenosis (MS) who want to become pregnant; in these women, preventive percutaneous treatment should be considered regardless of symptoms (Class IIA, level of evidence [LOE] C).
If an intervention is required during pregnancy, cardiac surgery should be avoided if possible due to the significant risk of cardiopulmonary bypass for the foetus, related to non-pulsatile blood flow and reduced uteroplacental flow.12,13 Coronary bypass surgery or valvular surgery may be considered during pregnancy only when conservative and medical therapy has failed, and in situations that threaten the mother’s life or that are not amenable to percutaneous treatment, which remains the first choice (Class IIb, LOE C).11
To date, an urgent or emergency structural intervention during pregnancy, could be required in the following clinical scenarios:
- Patients with high-risk VHD (mWHO Class IV) that was unknown at the time of conception (the incidence of this phenomenon is increasing due to migration flows) and when pregnancy interruption is refused.
- Patients with lower risk VHD, haemodynamic instability and refractory symptoms despite optimal medical therapy.
- Patients with acute onset of severe VHD during pregnancy (i.e. acute severe mitral regurgitation due to chordal rupture).
Percutaneous Mitral Balloon Valvuloplasty for Mitral Stenosis
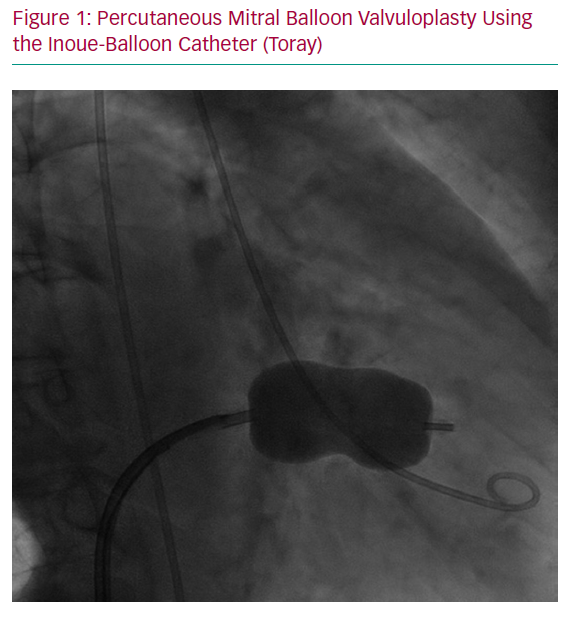
Percutaneous mitral balloon valvuloplasty (PMBV) for the treatment of selected patients with rheumatic MS was developed by Inoue et al. in 1984 and has revolutionised the treatment of this disorder (Figure 1).14,15 Rheumatic valvular disease is the most common cause of MS among women of childbearing age, particularly in low- to middle-income countries, but also in the developed world. Congenital heart disease (parachute mitral valve) is a less common cause of MS.
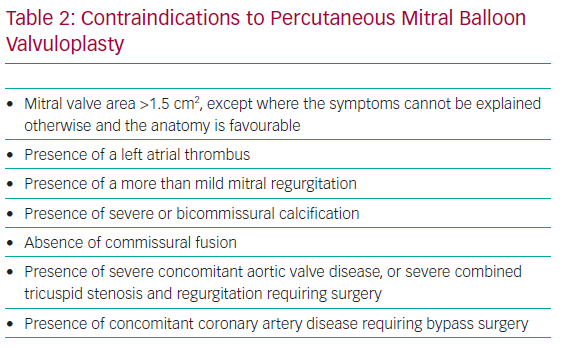
In pregnant women with MS, the increase in CO combined with a decrease in filling time due to increased heart rate can result in increased left atrial pressures, risk of AF and pulmonary oedema.16,17 Pregnancy in women with MS is associated with increased maternal morbidity and adverse foetal outcome. Accordingly, if severe MS is recognised before pregnancy, PMBV is recommended before conception, especially when the valve area is <1.0 cm2, if valve morphology is favourable for intervention, even if the patient is asymptomatic.11 If MS is recognised during pregnancy and haemodynamic compromise persists (New York Heart Association [NYHA] Class III/IV and/or pulmonary artery systolic pressure ≥50 mmHg) despite appropriate medical treatment (beta-blockers and diuretics), PMBV may be needed antenatally and is usually performed, unless contraindicated, during the second trimester (after the 20th week of pregnancy) in experienced centres.8 PBMV performed either before or during pregnancy seems to lead to equivalent maternal and foetal outcomes.18 PBMV was found to be superior to open mitral valve commissurotomy because it is a minimally invasive interventional procedure performed under local anaesthesia with significantly fewer foetal complications and reduced foetal and neonatal mortality compared with open surgery.19 PBMV is performed through percutaneous transvenous femoral access and requires transeptal puncture. Nonetheless, PBMV could be contraindicated in particular situations (Table 2).20 Other transcatheter technologies are being developed for patients with severe MS who are not eligible for PBMV due to unfavourable anatomy (e.g. very high Wilkins score or more than mild mitral regurgitation), such as percutaneous transeptal mitral valve implantation in mitral annular calcification (valve-in-MAC) or transcatheter dedicated mitral valve implantation.21,22 However, data and experience are lacking regarding the use of these techniques in pregnant women.
Transcatheter Balloon Aortic Valvuloplasty for Aortic Stenosis
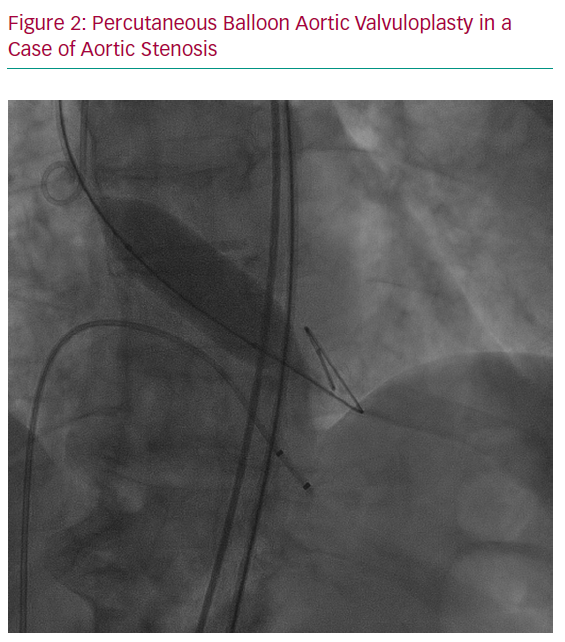
Congenital bicuspid aortic valve disease is the most common cause of aortic stenosis (AS) among women of childbearing age.23 Rheumatic heart disease is a less common cause of AS and is generally associated with rheumatic MS. All women with severe symptomatic AS should have a valve intervention before pregnancy because of the high risk of obstetric and foetal or neonatal complications.24 If AS is diagnosed, or becomes symptomatic, during pregnancy, valve intervention may be needed before delivery in case of refractory symptoms. In this context, and considering the high foetal risk of surgery, transcatheter balloon aortic valvuloplasty (BAV) can be undertaken by an experienced operator, depending on the exact morphology of the congenitally abnormal aortic valve (Figure 2).24,25 If the valve has clear commissures, is not thickened or calcified and is not regurgitant, cautious BAV, ideally with an undersized balloon to avoid aortic regurgitation, may provide a small increase in valve area, sufficient to allow the pregnancy to progress. However, compared with MS, data on BAV in pregnancy are scarce.26 When a BAV is considered by the pregnancy heart team, an associated aortopathy or aortic coarctation should be excluded, and the size of the ascending aorta should be always taken in account given the risk of aortic dissection.27 In addition, the feasibility of rescue surgical or percutaneous valve replacement should be confirmed before planning the intervention because emergency aortic valve replacement could be needed if severe aortic regurgitation develops.
Transcatheter Aortic Valve Replacement
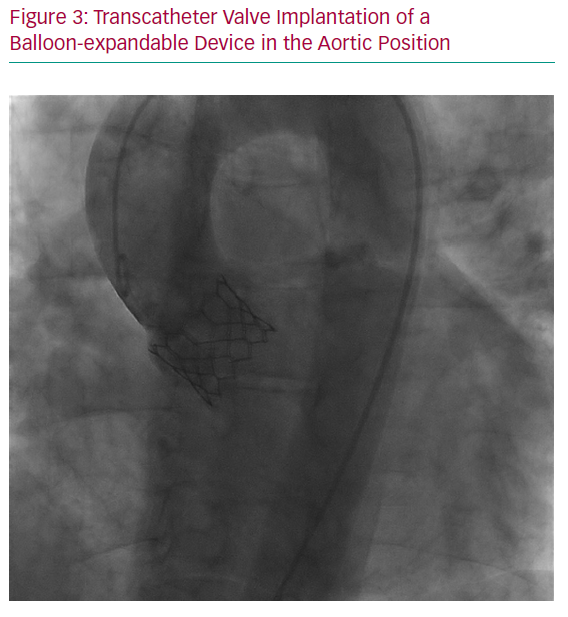
In patients with severe AS refractory to medical therapy, with unsuccessful BAV or associated aortic regurgitation, a transcatheter aortic valve replacement (TAVR) procedure, particularly through a femoral approach, seems to be a promising alternative to open heart surgery due to the perceived high likelihood of success with lower procedural risk for both the mother and foetus (Figure 3). Several transcatheter prostheses are now approved for clinical use, including in low-risk patients. However, experience with TAVR during pregnancy is still anecdotal, and key to the success of this procedure is a case-by-case evaluation and discussion by the heart team.2,8 Preprocedural assessment, which implies contrast medium injection and radiation, should be performed with caution, and transoesophageal echography could help assess valve size and anatomy while avoiding radiation and contrast medium. The other main difficulty in young women is the absence of calcification in this age group, which may preclude optimal positioning and stability of the prosthetic valve. Moreover, the risk of heart block and the need for a pacemaker should be carefully evaluated and discussed with the patient, as well as the lack of robust data about long-term durability of transcatheter prostheses in such young patients.
Percutaneous Mitral Valve Repair
Acute mitral regurgitation, due, for example, to a ruptured chord, is not well tolerated during pregnancy. In addition, chronic mitral regurgitation can become symptomatic during pregnancy. In case of refractory symptoms despite diuretic therapy, transcatheter mitral valve repair (edge-to-edge repair or annuloplasty) could, theoretically, be an intriguing solution, reducing maternal and foetal risk by avoiding extracorporeal circulation, even though no data have been published so far. As noted above, transcatheter mitral valve replacement is a solution under investigation but, to date, no data are available in pregnant women.22 All these procedures are generally performed under general anaesthesia, through transapical or transvenous femoral access followed by transeptal puncture, and with transoesophageal ultrasound guidance.29,30
Transcatheter Valve-in-Valve Implantation in Degenerated Bioprosthesis
Until few years ago, the treatment of bioprosthesis degeneration leading to severe stenosis or regurgitation was a real challenge in pregnant women with symptoms refractory to medical therapy. In fact, redo open heart surgery was the only therapeutic option.
The recent widespread use of transcatheter valve-in-valve procedures has provided an important alternative option in these cases, with high procedural success rates and very low risk with respect to surgical redo. Therefore, despite the small amount of data during pregnancy, the valve-in-valve procedure already seems to be the optimal therapy for symptomatic pregnant women with severe bioprosthesis dysfunction in aortic and other anatomical locations.31–34 However, there are no data regarding the safety and durability of such a procedure in young women. Moreover, due to the high prevalence of small aortic root anatomies in young women, the risk of prosthesis–patient mismatch in case of valve-in-valve implantation should be carefully evaluated.35 Again, contrast medium and radiation should be used with caution during pregnancy (see below).
Considerations Regarding Radiation Exposure
The general principles for imaging during pregnancy are similar to imaging in the general population, with the goal of keeping radiation exposure as low as reasonably achievable. As obvious ethical issues prohibit researching on the foetus, most of the data on the effect of radiation on the foetus derives from observations made of victims of high-level radiation exposure.
The effect of radiation exposure during pregnancy depends on both radiation dose and the gestational age of the foetus. By the second trimester of pregnancy, organogenesis is complete and the foetal thyroid is still inactive. Accordingly, if an intervention is absolutely necessary, the best time is after the fourth month in the second trimester. Moreover, in the second trimester, the uterine volume is still small, so there is a greater distance between the foetus and the chest than in later months; therefore, there is no reason to postpone a necessary intervention until later. According to the Centers for Disease Control and Prevention, a foetal radiation dose <50 mGy is considered safe and harmless. Higher doses, especially doses >150 mGy, may result in adverse effects, including miscarriage, growth reduction, IQ reduction and severe mental retardation.36 Interestingly, the majority of diagnostic studies performed during pregnancy are below the toxicity threshold. However, every effort must be made to minimise radiation exposure by weighting the risk versus benefit of each intervention, referring these patients to experienced interventional cardiologists and adopting radiation reduction measures (Table 3).11,37,38
It is essential to remember that catheter-based diagnostic and interventional studies should not be avoided for fear of radiation exposure, especially when these studies can dramatically change maternal and foetal management and improve outcomes.
In particular, current evidence suggests that a single cardiovascular imaging study during pregnancy is safe and should be undertaken at all times when clinically justified.
Considerations Regarding Anticoagulation Therapy During Pregnancy
Oral Anticoagulants
Direct oral anticoagulants (DOACs) are contraindicated throughout pregnancy;39 thus, vitamin K antagonists (VKAs) remain the most effective anticoagulant regimen. However, VKAs cross the placenta and their use in the first trimester can result in embryopathy (limb defects and nasal hypoplasia) in 0.6–10% of cases.40 Moreover, as described in the Registry of Pregnancy and Cardiac Disease (ROPAC),41 the use of VKAs versus heparin in the first trimester was associated with a higher rate of miscarriage (28.6% versus 9.2%, respectively; P<0.001) and late foetal death (7.1% versus 0.7%, respectively; P=0.016). This risk seems to be dose dependent, and recent reviews suggest that is <1% with low-dose warfarin (<5 mg daily).42–44 Apart from this risk of embryopathy that is limited to the first trimester, the use of VKAs in the second and third trimesters can lead to a 0.7–2% risk of foetopathy (e.g. ocular and central nervous system abnormalities and intracranial haemorrhage).40 Moreover, VKAs must be discontinued before vaginal delivery because of the risk of foetal intracranial bleeding.40
Unfractionated Heparin
Unfractionated heparin (UFH) does not cross the placenta, so its use in Weeks 6–12 is safe, and almost eliminates the risk of embryopathy when replacing VKAs.3 However, foetopathy has also been described with UFH throughout pregnancy, so its use during the second and third trimesters is not recommended.43 The main disadvantages of UFH are the higher risks of osteoporosis and thrombocytopenia, requiring platelet counts every 2–3 days.45 Therefore, the use of UFH is limited to the acute treatment of massive pulmonary emboli and around the time of delivery, when the ability to reverse anticoagulation urgently using protamine is advantageous. UFH remains the preferred antithrombotic regimen in the catheterisation laboratories during catheter-based interventions, where UFH has to be given intravenously at a dose of 40–70 U/kg targeting an activated clotting time of 250 s (200–300 s) or an activated partial thromboplastin time twice that of normal.
Low-molecular-weight Heparin
The efficacy and safety of several low-molecular-weight heparin (LMWH) preparations have been well demonstrated in pregnant women.46,47 The main advantage of LMWH is its safety in weeks 6–12, when its use instead of VKAs almost eliminates the risk of embryopathy; in addition, no case of foetopathy has been described with LMWH throughout pregnancy.43,44 Moreover, in contrast to UFH, heparin-induced thrombocytopenia is markedly lower with LMWH, as is heparin-induced osteoporosis.45 Conversely, monitoring is essential to maintain a certain therapeutic anti-Factor Xa level, in particular in patients with mechanical valves or when pulmonary embolism has occurred in women receiving prophylactic doses of LMWH.48 Considering this unfavourable pharmacokinetic feature, LMWH should be switched to intravenous UFH 36 hours before the induction of labour or when caesarean delivery is planned in order to reduce haemorrhagic complications.
Fondaparinux
Fondaparinux is a direct inhibitor of Factor Xa activity via antithrombin III binding. Given the scarce evidence on the use of fondaparinux in pregnancy, together with possible minor transplacental passage, its use should be limited to cases of documented allergy or adverse response to LMWH. More data are required to assess the risk of congenital malformations with fondaparinux.49,50
Practical Use of Anticoagulation Therapy During Pregnancy
Native Valve Disease
Systemic embolisation may occur in up to 10–20% of patients with MS, with the highest risk in patients with AF. Pregnancy is associated with a hypercoagulable state and is expected to further increase the risk of thromboembolic events. Therefore, immediate anticoagulation is required in all patients with MS and AF. The suggested regimen in such cases is LMWH at therapeutic doses in the first trimester and VKAs with the usual target international normalised ratio (INR; or LMWH) for the second and third trimesters. These patients should then be converted to continuous infusion of UFH before planned delivery.11
Cardioversion seems safe in all phases of pregnancy, but the choice to do it depends on the tolerance of the patient and on the severity of the underlying valve disease.51
Mechanical Heart Valves
Anticoagulation of mechanical heart valves is also a challenge in pregnant patients, and current evidence, even though randomised studies are lacking, tends to indicate that VKAs with a strictly controlled INR are the safest treatment to avoid valve thrombosis throughout pregnancy. For example, VKAs should be continued throughout pregnancy and replaced by UFH at 36 weeks of gestation, particularly when the required VKA dose is low (warfarin <5 mg/day, phenprocoumon <3 mg/day or acenocoumarol <2 mg/day), because of the low risks of embryopathy, foetopathy (<2%) and foetal loss (<20%) and because VKAs are the most effective regimen to prevent valve thrombosis.
When a higher dose of VKAs is required, discontinuation of VKAs between weeks 6 and 12, and replacement with adjusted-dose intravenous UFH or LMWH twice daily with dose adjustment according to peak anti-Factor Xa levels, should be considered. The use of LMWH remains controversial due to the higher risk of valve thrombosis that is mitigated by a strict control of anti-Factor Xa levels. The treatment must be discussed with the patient and individualised, especially in terms of VKA dosage, according to the stage of pregnancy (with the first trimester to be considered on its own), the patient’s compliance and the type of prosthesis.11,43
Conclusion
In conclusion, VHD is a relatively rare condition in pregnant woman, but should be carefully managed due to the risk of cardiac decompensation and worsening prognoses for both the mother and foetus. A multidisciplinary pregnancy heart team should be involved in any clinical steps, from prepregnancy counselling to management during pregnancy and around delivery, in order to anticipate and potentially avoid complications.
Evolving technologies in the field of interventional cardiology have been developed, allowing, in many cases, a minimally invasive transcatheter treatment of VHD. The risk–benefit ratio for both the mother and foetus must be considered when planning these procedures, and every effort must be made to minimise the risks.