Dr. Scheiber is a practicing cardiac physician at Heinrich Heine Universität Düsseldorf. He was the recipient of the Young Investigator Scholarship for the 2017 A-CURE Symposium.
Dr Scheiber was the recipient of the Young Investigator Scholarship Award. He commenced his presentation with a discussion of mitochondrial energy metabolism in the failing heart. The heart consumes more energy than any other organ. To match this high energy demand, myocardial mitochondria cycle up to 6 kg of ATP every day, which is about 20 to 30 times the heart’s own weight. Myocardial mitochondrial energy metabolism in the failing heart has, therefore, become a field of considerable interest.1,2
Increased ventricular filling pressure and volume are hallmarks of heart failure (HF) pathophysiology.3,4 This pressure and volume overload is linked to alterations in myocardial substrate utilisation, mitochondrial energy production, and mitochondrial reactive oxygen species formation.5,6 Clinical evidence suggests that ventricular unloading can reverse systemic and local metabolic dysfunction in patients with advanced HF treated with durable ventricular assist devices.7 However, there are no functional data on mitochondrial respiration in the failing heart under these unloading conditions.
Dr Scheiber’s team proposed the idea that chronic left ventricular unloading in terminal patients with HF would improve myocardial mitochondrial oxidative phosphorylation and reduce myocardial mitochondrial reactive oxygen species production. In order to investigate this hypothesis, a prospective study evaluated 13 patients undergoing heart transplantation between October 2016 and July 2017. Eight patients had a left ventricular assist device (LVAD) surgically implanted as a 'bridge to transplant' prior to heart transplantation. Myocardial tissue specimens were harvested from macroscopically scar-free areas of the left ventricular free wall of the explanted heart. Patients did not differ significantly in age or body mass index. The average time of unloading in the LVAD-supported patients was 20 months.
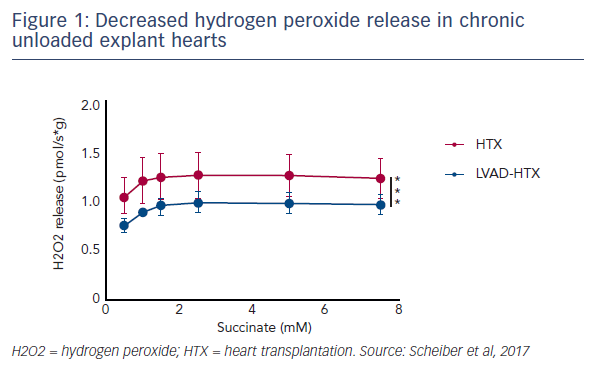
The ex vivo maximal myocardial oxidative phosphorylation capacity was analysed in tissue specimens. There was a similar maximum oxygen flux on fatty acids and tricarboxylic acid cycle derivates in chronically unloaded compared with standard heart explants. Interestingly, the respiratory control ratio, which is a surrogate marker of coupling efficiency, was significantly increased in the unloaded group compared with the standard transplant group, suggesting more efficient ATP production in these mitochondria. Analysis of myocardial mitochondrial hydrogen peroxide production between these two groups showed that reactive oxygen species production during mitochondrial respiration was decreased in tissue samples of the chronic unloaded hearts (see Figure 1).
In conclusion, this study found an increased mitochondrial coupling efficiency and decreased hydrogen peroxide production in chronically unloaded hearts, but no differences in maximal mitochondrial respiration when comparing those hearts haemodynamically unloaded by LVADs with hearts that were unsupported. Future research will investigate whether alterations of mitochondrial respiration occur during ventricular unloading in acute cardiogenic shock and, if so, how this may impact patient outcomes and heart recovery. Other planned studies include the impact of acute ventricular unloading on mitochondrial respiration and hydrogen peroxide production.