Acute coronary syndrome (ACS) is the most common form of coronary heart disease and is a leading cause of mortality worldwide.1–3 Although successful angioplasty has significantly increased survival among ACS patients, the ‘no-reflow’ phenomenon, where myocardial perfusion is ineffective despite angiographic success occurs frequently and is related to an increased risk of mortality.4,5
Angiographic success is defined as a thrombolysis in MI (TIMI) flow grade of 3, and a minimum stenosis reduction of at least 50% for balloon dilatation or at least 10% reduction for coronary stenting.6–10 However, studies show that, although epicardial TIMI 3 flow grade is restored in 80–90% of patients after angioplasty, adequate myocardial perfusion is achieved less frequently and detrimentally impacts patient survival.11–14
In a cohort study by Stone et al., normal epicardial flow was restored in 94.2% of patients but only 29.4% with TIMI 3 flow grade had normal myocardial perfusion.12
In a later study by Constantino et al., 81% of patients who achieved a TIMI flow grade of 3 after angioplasty had reduced myocardial perfusion; these patients had significantly reduced survival, with 1-year mortality rates of 4.1% for patients with reduced perfusion and 6.2% for those with absent perfusion compared to the 1.4% mortality rate for patients with normal perfusion (p=0.01).14
Myocardial perfusion can be assessed in many ways but the most studied of these is myocardial blush grade (MBG). First described by van’ t Hof et al. in 1998, MBG is determined on the angiograms made immediately after primary coronary angioplasty using the best projection angles to assess the myocardial region of the infarct-related coronary artery.11 Unlike TIMI flow grade, which evaluates blood flow along the main epicardial artery, MBG evaluates the microvascular patency of the distal capillaries perfusing the myocardium.15
MBG is defined as follows: 0 – either no myocardial blush or persistent myocardial blush ‘staining’; 1 – minimal myocardial blush or contrast density; 2 – moderate myocardial blush or contrast density, but less than that obtained during angiography of a contralateral or ipsilateral non-infarct-related coronary artery; and 3 – normal myocardial blush or contrast density that is comparable with that obtained during angiography of a contralateral or ipsilateral non-infarct-related coronary artery (Supplementary Material Table 1).11,12
Several studies have reported a good correlation between MBG and markers of post-infarction myocardial function such as contrast-enhanced cardiac MRI, contrast echocardiography and post-angioplasty QRS duration change.16–18 Some studies have also reported MBG to correlate well with clinical outcomes.14,19
Therefore, this study aims to synthesise the evidence on the role of MBG as a predictor of all-cause mortality and major adverse cardiovascular events (MACE) among acute coronary syndrome patients who underwent primary angioplasty.
Objectives
The study aimed to evaluate the impact of post-angioplasty MBG on the mortality of patients with ST-elevation MI (STEMI).
Methods
This systematic review and meta-analysis used the PROGRESS (PROGnosis RESearch Strategy) framework and is reported in line with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guide. Eligibility criteria were formed using the PICOTS (Patient, Intervention, Comparison, Outcome, Time and Setting) framework and data extraction was done using CHARMS-PF (CHecklist for critical Appraisal and data extraction for systematic Reviews and prediction Modelling studies for Prognostic Factors).20
Eligibility
All applicable studies based on the PICOTS of this study were included:
- population – adults who underwent primary coronary angioplasty for STEMI;
- index/intervention – MBG;
- comparison groups – MBG 0/1 versus MBG 2/3, MBG 2 versus MBG 0/1 and MBG 3 versus MBG 0/1;
- outcome – all-cause mortality/MACE at follow-up;
- timing – follow-up of at least 12 months; and
- setting – observational studies and randomised trials in single centre or multicentre settings.
Studies were excluded for the following reasons:
- the full article was not available;
- narrative review or editorial;
- MBG was not obtained immediately after the primary percutaneous coronary intervention (PCI) or at the end of the procedure; or
- the study did not measure mortality or MACE.
Search
The search used topic-based strategies designed for each database. There were no language or geographic restrictions. The publication search was limited to systematic reviews, meta-analyses, observational studies and randomised controlled trials published from 1998 to 2021. The reference lists of included articles were hand searched to identify additional studies.
The PubMed, ScienceDirect and Cochrane research databases were used, as was the Google Scholar search engine.
The following keywords and corresponding MeSH terms, derived from a scoping search and expertise in the subject field, were used for the systematic search: [“myocardial blush grade” AND (“acute coronary syndrome” OR “acute myocardial infarction”)] were indexed to the research databases (Supplementary Material Table 2).
Articles for screening were stored into a computer document folder and data collected were encoded into an Excel file.
Article Selection, Data Collection and Assessments
Two researchers (PVC and NB) independently performed the database search, assessed studies for for applicability and eligibility, collected data, assessed risk of bias and decided which studies should be included. If there were inconsistencies, a third researcher (PPP) was consulted to make a decision.
Each included article was assessed for relevance of reported data. A study was determined to be relevant if the population being studied included patients who underwent PCI due to STEMI, data on post-angioplasty MBG, data on the event rates of all-cause mortality and MACE (defined as a composite of death, MI, heart failure and ischaemia-driven revascularisation after a follow-up of at least 12 months) were reported.
Data on methods to minimise or determine inter- and intra-observer disagreement were also collected.
Effect Measures and Additional Analyses
To determine the cumulative prognostic value of MBG 0/1, the odds ratios for all-cause mortality and for MACE with MBG 0/1 were obtained from each included study and analysed. Sensitivity analysis for the prognostic value of MBG 0/1 was also performed by removing studies that did not report adjusted ORs as an outcome measure. To determine the prognostic value of MBG 2 and MBG 3, the HRs for mortality were obtained from all included studies and analysed.
WebPlotDigitizer 4.4 was used to construct Kaplan-Meier curves from studies that did not report numerical effect measures to obtain the logarithmic hazard ratios following the methods described by Tierney et al. and Wei et al.21–23 A prespecified subgroup analysis was also done for studies that included patients in cardiogenic shock or were haemodynamically unstable to reduce heterogeneity.
RevMan 5.4 was used to create figures to display the results of individual studies and forest plots of the meta-analyses in a Mantel-Haenszel fixed effects model. The I2 test and the τ2 (DerSimonian and Laird) methods were used to determine degree of heterogeneity between the individual studies.
The Quality In Prognosis Studies (QUIPS) tool was used to evaluate the risk of bias of each study. To assess for publication bias, a funnel plot was constructed to identify possible publication bias. The Begg and Mazumdar rank correlation and the Egger’s regression intercept were used to examine asymmetry.
Certainty Assessment
The strength of the overall body of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework for systemic reviews and meta-analyses of prognostic factors.24
The GRADE approach considers five factors that can decrease confidence in estimates of effects: study design and limitations in study design; inconsistency of results across studies; indirectness of the evidence; imprecision; and publication bias; and three factors that can increase confidence in estimates of effects from observational studies: large estimates of treatment; a dose-response gradient; and plausible confounding that would increase confidence in an estimate.
The starting point for the quality level of the evidence is based on the phase of investigation. The level of certainty is then upgraded or downgraded depending on the GRADE factors.
The results of assessment of certainty were presented in a summary of findings table.
Results
Article Search and Selection
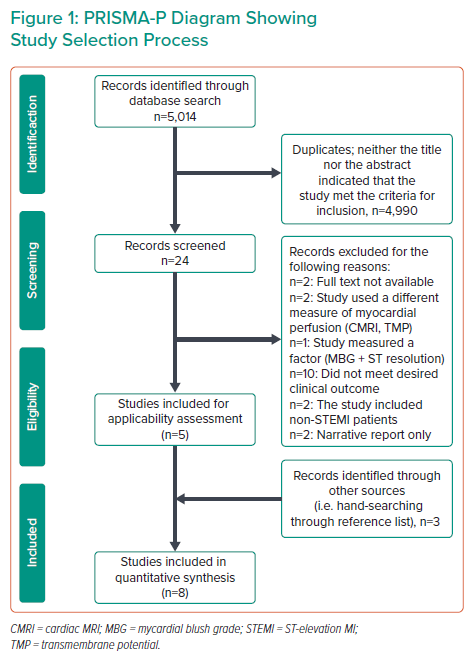
A total of 5,014 records were identified from PubMed (n=115), Cochrane (n=209), ScienceDirect (n=530) and Google Scholar (n=4,160; Figure 1). No restrictions were made regarding language. After exclusion of narratives, editorials, duplicates and studies where neither the title nor the abstract indicated the parameters for inclusion, 24 records were screened.
Research papers where the full text was not available (n=2), the population included non-STEMI patients (n=2), a different measure of myocardial perfusion was measured (n=2), MBG was not analysed independently (n=1), outcomes did not include mortality or MACE (n=10) and narrative reports (n=2) were excluded.
Authors were contacted via email to request full texts of their research papers and for other relevant information pertaining to their study. Three articles were added after hand-searching the reference list of the seven articles eligible for inclusion, making a total of eight articles that qualified according to the inclusion and exclusion criteria for this meta-analysis.
Study Characteristics
Supplementary Material Table 3 shows the study characteristics of the included articles in this systematic review. All articles included were prospective observational studies, involving a total of 8,044 STEMI patients. All the studies measured MBG after primary PCI. Outcome measures in all the included studies included all-cause mortality and MACE follow-up time to all studies being 1 year.11,13,14,18,19,25,26
Risk of Bias Assessment
The QUIPS tool was used to assess the risk of bias of each study (Hayden et al.).27 The QUIPS tool uses six domains: study participation; study attrition; prognostic factor measurement; outcome measurement; study confounding; and statistical analysis and reporting. Each domain includes multiple items that are judged separately.
Based on the rating of the included items, a conclusive judgement of the risk of bias within each domain was made and expressed on a three-grade scale (high, moderate or low risk of bias). All 10 studies included had an overall low risk of bias (Supplementary Material Table 4).
Study Participation
All articles involved STEMI patients only.11,13,14,18,19,25 They all adequately described the populations, and clinical and demographic factors that were significantly different between the two groups compared were identified. There were also no identified significant deviations from the in-study definitions of STEMI. However, the study by Brener et al. was able to enrol only 71% of the eligible population so was determined to have a moderate/unclear risk of bias.28
Study Attrition
Only the study by Kampinga et al. had a <100% follow-up rate, but they declared their follow-up rate was >99% among the 2,118 STEMI patients they enrolled.19 Hence, all the studies included were determined to have a low risk of attrition bias.
Prognostic Factor Measurement
All studies had similar definitions for MBG as described by Van ’t Hof et al.11 However, the study by Kampinga et al. showed a moderate inter-operator agreement with a κ coefficient of 0.47, so the studies were determined to have a moderate/unclear risk of bias.19
Study Confounding
All studies took possible confounding into account appropriately by using multivariate analysis and Cox regression statistics for determining effect measures and associations. Hence, there was a low risk of bias for possible confounding variables.
Statistical Analysis and Reporting
Five of the included studies did not report adjusted OR for the association between MBG 0/1 and mortality.13,14,18,26,28 Hence, these studies were determined to have an moderate/unclear risk of reporting bias.
To explore the effect of this potential bias, sensitivity analysis was done by performing a separate analysis using only the adjusted odds ratios of MBG 0/1 for mortality.
Certainty of evidence
As seen in Supplementary Material Tables 5, 6 and 7 in the supplementary data, this systematic review and meta-analysis has a high overall certainty of evidence using the GRADE framework for the prognostic value of MBG 0/1 and MBG 3.
Publication Bias
There was no indication of publication bias among the studies after assessment with Begg and Mazumdar rank correlation (Kendall τ 0.122; p=0.464) and Egger’s regression (p=0.285).
Main Results of the Meta-Analysis
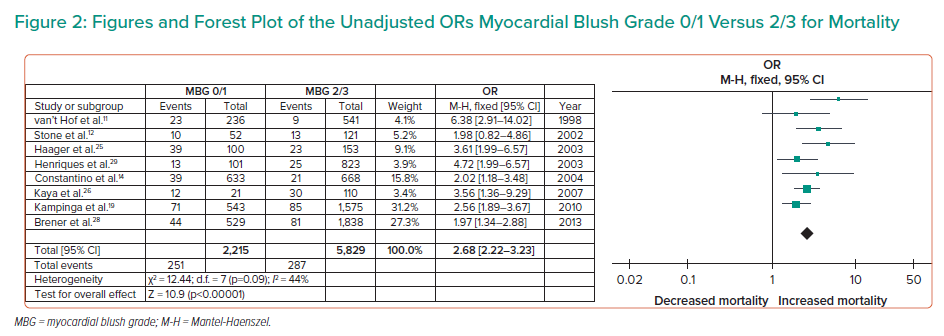
Pooled results of eight observation studies involving a total of 8,044 patients with STEMI were included in this meta-analysis. All included studies had an overall low risk of bias. With a high certainty of evidence, there was significant association between MBG 0/1 and all-cause mortality among patients with STEMI (unadjusted OR 2.68; 95% CI [2.22–3.23]; τ2 0.05; I2 44%; Figure 2).
After separately analysing only the adjusted OR of MBG 0/1 for mortality in a sensitivity analysis, the cumulative association of MBG with mortality was still significant (adjusted OR 2.29; 95% CI [1.97–2.67]; τ2 0.00; I2 0%; Supplementary Material Figure 1).
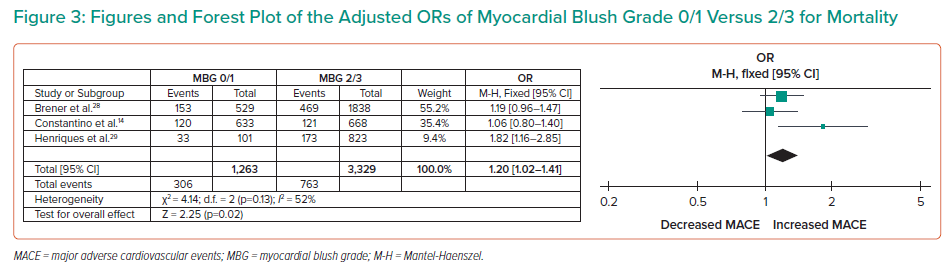
MBG also showed a significant association with MACE, defined as a composite of death, MI and ischaemia-driven revascularisation (OR 1.20; 95% CI [1.02–1.41]; τ2 0.02; I2 52%) with a high level of certainty of evidence (Figure 3).
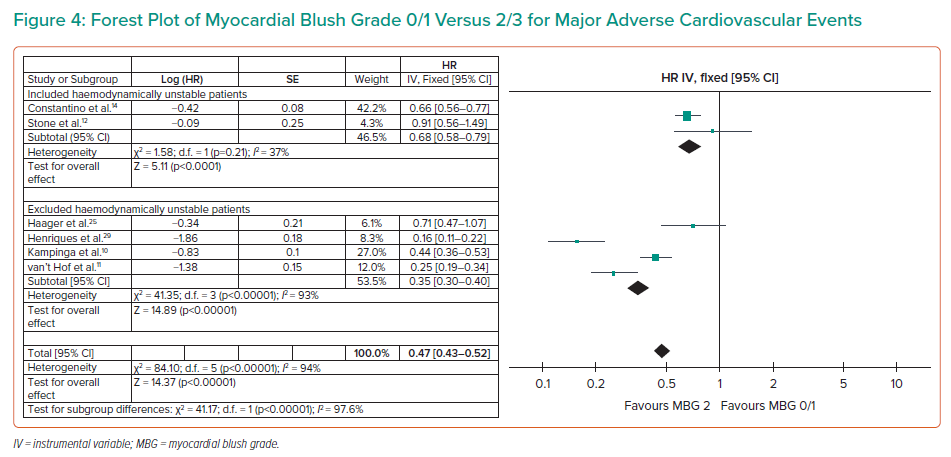
An MBG of 2 (moderate myocardial blush) showed a strong association with survival compared to an MBG of 0/1 (HR 0.47; 95% CI [0.43–0.52]; I2 94%) (Figure 4).
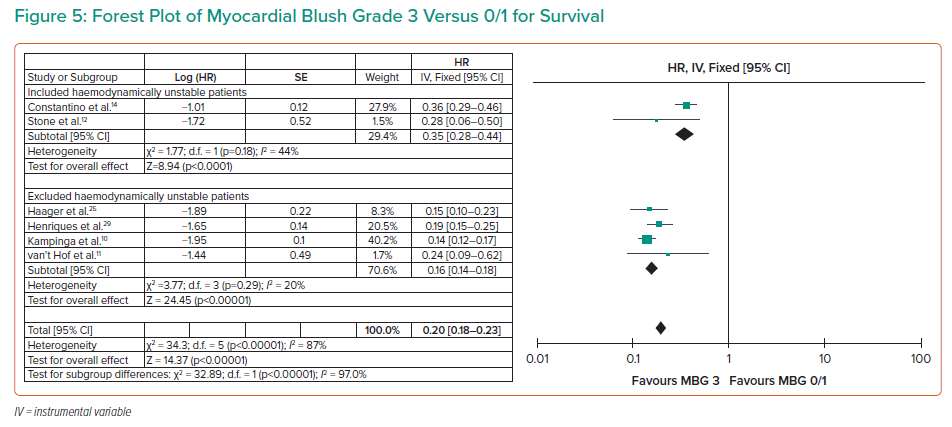
A stronger association with survival was observed with an MBG of 3 (HR 0.20; 95% CI [0.18–0.23]; I2 89%; Figure 5). With a high level of certainty, this association was consistent after subgroup analysis for studies that included haemodynamically unstable patients (HR 0.35; 95% CI [0.28–0.44]; I2 44%) and those that excluded them (HR 0.16; 95% CI [0.14–0.18]; I2 20%) with a p-value for subgroup differences <0.001 at 95% CI.
Discussion
After a systematic search and review of 5,031 articles, the researchers found no other meta-analysis examining the prognostic value of MBG for post-angioplasty ACS patients.
This study shows that an MBG of 0/1 with minimal to no myocardial perfusion has a robust negative prognostic value for all-cause mortality among patients with STEMI. A similar association was present between MBG 0/1 and MACE. The pathophysiologic mechanism behind poor perfusion despite adequate epicardial blood flow has been discussed in several studies as secondary to distal microthromboembolism that prevents myocardial perfusion after angioplasty.7,29–31 Unfortunately, there is currently no established treatment or prevention for this phenomenon but pharmacological and catheter-based approaches have been proposed.32,33
This meta-analysis also shows that an MBG of 2 or 3 is a strong positive prognostic marker for survival at follow-up of at least 12 months. These findings emphasise the need to ascertain myocardial perfusion status after angioplasty and support the relevance of determining MBG as a surrogate marker for predicting long-term survival among STEMI patients after angioplasty. This should be taken into account along with other markers of long-term survival, such as ischaemic time, infarct size and thrombotic burden.
Limitations
Since majority of the included articles did not publish numerical data for effect measures, the unadjusted HRs were indirectly obtained from Kaplan-Meier curves using the methods by Tierney et al.21 Another limitation of this study is that the search for articles was limited to research databases, so relevant but unpublished articles presented at scientific conferences may have been missed.
Lastly, MBG is a surrogate marker for survival and various other factors also contribute to a patient’s long-term outcomes, such as prolonged ischaemia time, infarct size and thrombotic burden.
Recommendations
We recommend further studies to determine the usefulness of MBG assessment for unstable angina/non-STEMI patients since the data gathered in this study was not enough to draw a definite conclusion. Furthermore, modifications of MBG to make the assessment of myocardial perfusion more quantitative have also been suggested. Hence, studies comparing the reproducibility using these methods would also be prudent. We also recommend that interventional studies addressing no reflow after angioplasty to target a MBG of 3 or its equivalent are carried out, since this is strongly associated with increased long-term survival.
Conclusion
This systematic review and meta-analysis shows that a post-angioplasty MBG of 0/1 is a strong negative prognostic marker for mortality and MACE and, on the other side of the spectrum, an MBG of 2 or 3 is strong positive prognostic marker for survival among STEMI patients.
Click here to view Supplementary Material.
Clinical Perspective
- This study shows that myocardial blush grade (MBG) is robustly associated with patient outcomes. Hence, management guidelines may consider MBG as an additional angiographic parameter to define angiographic success.
- By determining MBG, clinicians can also better prognosticate patients and decide on management plans to improve long-term patient outcomes.
- Future studies may consider using MBG to evaluate the efficacy of treatment modalities that focus on the prevention and treatment of poor myocardial reperfusion after primary angioplasty.