Dr Tedford opened his talk with a patient case: a 62-year-old man with non-ischaemic cardiomyopathy with a left ventricular (LV) end-diastolic diameter of 7.7 cm and a LV ejection fraction of 25%. The patient had severe mitral regurgitation and a dilated right ventricle (RV) with mild to moderate dysfunction and moderate tricuspid regurgitation. His renal and liver functions were normal and he had elevated pulmonary vascular resistance but was not on any inotropes. The patient was treated with nitroprusside. His Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile was 4. The patient’s right arterial pressure (RAP) was normal at 6 mmHg; pulmonary artery wedge pressure (PAWP) was elevated at 20 mmHg and his pulmonary artery pulsatility index (PAPi) was 3.33. These parameters did not signify a high risk of RV failure (RVF) and this patient would not be considered at high risk of RVF after implantation of a durable left ventricular assist device (LVAD) according to traditional criteria. However, the patient ended up experiencing acute RVF following HeartMate 3 implantation. This case study illustrates how hard it is to predict RVF prior to implant and yet RVF following LVAD implantation continues to be associated with poor outcomes.
Dr Tedford outlined the traditional methods used to assess RVF, which include an array of haemodynamic, echocardiographic and MRI metrics (Table 1). However, no single metric in isolation is a good predictor to tell which patients will develop RV dysfunction.
In further efforts to predict RVF, a considerable number of risk scores have been developed but independent validation cohorts have shown that these are also poor at predicting RVF.1,2 The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) Right Heart Failure (RHF) risk score with a large derivation and validation cohort outperformed other risk scores and clinical predictors of early postoperative RHF, but even so, the C-index of the composite score was 0.70 in the derivation and 0.67 in the validation cohort.3
As an example, when various risk scores are applied to the opening patient case, none would have predicted RVF, with scores of 0 (low risk) on EUROMACS RHF, 0 (low risk) on the postoperative EUROMACS RHF, 0 (low risk) on the Kormos RV risk score and 1 (low risk) on the CRITT RV score.
Despite these ongoing efforts to predict and prevent RHF, it remains a leading cause of morbidity and mortality in heart failure patients, particularly after implantation of a durable LVAD. It is particularly challenging because multiple clinical factors and therapies impact RV function in pre-LVAD patients. Dr Tedford assembled a perspective in 2017 to summarise the potential mechanisms of RV dysfunction after implantation of a durable LVAD, including the decline in septal function from the reduced twist of the heart and a decline in RV contractibility due to LV unloading.4
The gold standard to assess RV function is pressure–volume analysis, but this is an invasive procedure and not always practical. Dr Tedford referred to a number of studies that aimed to identify RV function indicators. One study performed on a piglet model of pulmonary hypertension investigated whether RV–pulmonary artery coupling could be related to RV reserve.5 That study found that RV reserve was associated with ventricular–arterial coupling.5 Another clinical study showed that RV ejection fraction during submaximal exercise is an index of RV contractile reserve, and provides a better identification of RV dysfunction than resting measures.6 Finally, a study in six patients with biventricular failure who underwent dobutamine stress echocardiography within 30 days of mechanical circulatory support (MCS) implant showed that individuals who did not develop RHF had higher systolic pulmonary artery pressure and tricuspid annular plane systolic excursion that those who did.7 As a result of these efforts, a step was taken to redefine contractile reserve as the response of the RV to LV unloading with pharmacological (vasodilator) or temporary mechanical support.8
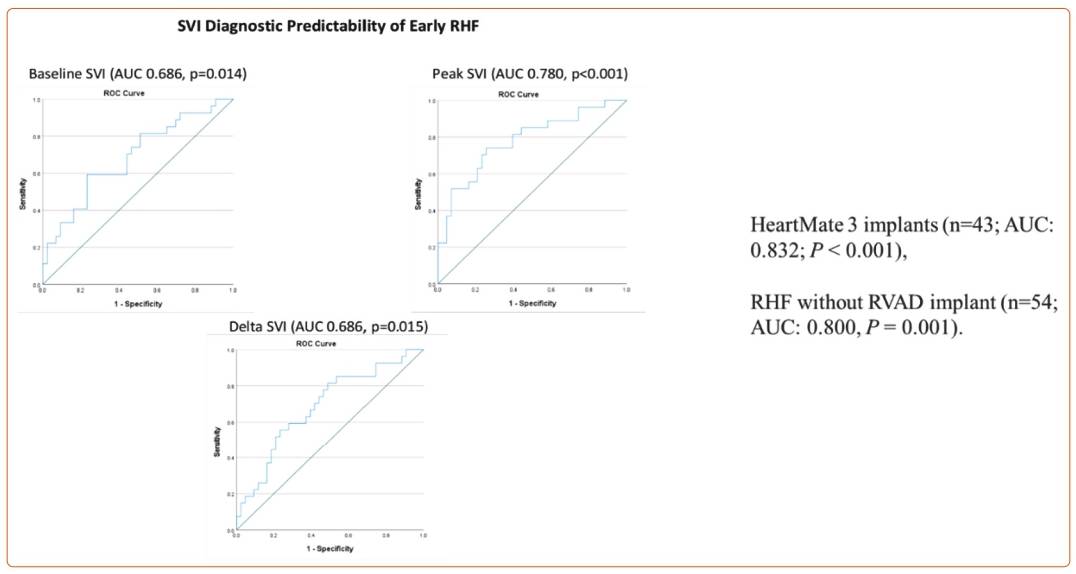
Using this definition, Dr Tedford presented an overview of his retrospective multicentre study performed on patients undergoing durable LVAD implantation.9 Seventy patients underwent right heart catheterisation (RHC) and vasodilator testing with nitroprusside as part of LVAD/transplant evaluation. Most patients had combined post- and precapillary pulmonary hypertension. The 2020 MCS Academic Research Consortium definition was used to define RHF,10 and a validation cohort of 10 consecutive patients was included. Of these patients, 39% met criteria for RVF and 20% required a right ventricular assist device, half of which were planned. Baseline demographics, INTERMACS profiles, haemodynamics, echo parameters and laboratory parameters were similar between those who did and did not develop RHF. There was no difference between the most used RHF risk scores, including the EUROMACS RHC score. The results of Dr Tedford’s study showed that the difference in stroke volume index (SVI) in response to nitroprusside, and specifically peak SVI, was a better indicator of patients who went on to develop RHF compared with baseline SVI alone, as shown in Figure 1. If SVI was maintained above 22.10 ml/m2, the likelihood of the patient developing RHF was low.9
Another recent study conducted at a single centre also found that SVI varied during peak nitroprusside infusion.11 This investigation additionally found that post-nitroprusside PAPi was improved in those patients who did not develop RHF. SVI and PAPi could provide independent predictors of RVF following LVAD implantation. In addition, Gonzalez et al. looked at the peak haemodynamic parameters to predict RVF and found that optimal PAPi was the best predicator of RVHF.12 Hsi et al. examined the RV response by unloading with an Impella device and found that individuals with a significant decrease in right atrial pressure, or no increase in the RAP/PAWP ratio or an improvement in PAPi were less likely to develop RVF after LVAD implantation.13
Returning to the patient case described at the beginning of his presentation, Dr Tedford showed that after nitroprusside infusion the SVI was low at 19.8 ml/m2 and PAPi decreased from 3.33 to 2.0. These parameters suggest the patient had a lack of RV reserve prior to LVAD implantation.
Dr Tedford concluded his presentation by summarising that RV reserve is associated with the gold-standard measure of RV function, and vasodilator testing (or temporary MCS) to unload the LV may be another way to assess RV reserve and predict RV failure in patients after they undergo LVAD implantation. He acknowledged that prospective studies are needed to test these hypotheses.