MI with non-obstructive coronary arteries (MINOCA) is a heterogeneous group of vascular or myocardial disorders that was first reported over 80 years ago.1 MINOCA is not a benign diagnosis, with outcomes similar to those of patients with acute MI and obstructive coronary disease up to 1 year (12-month mortality 0.6% versus 2.3%, respectively; p=0.68).2,3 MINOCA occurs in 5–15% of patients presenting with acute ST-segment elevation MI (STEMI) or non-ST segment elevation MI (NSTEMI), depending on the observed population and definition used.4,5 Compared with obstructive coronary artery disease, factors associated with MINOCA include female sex, younger age (<55 years), genetics and physiological stress.6–8 Accurate diagnosis and subsequent management require the appropriate utilisation of intravascular imaging and coronary function testing, in addition to echocardiographic and cardiac MRI (CMR) to assess for the presence of infarction or myocardial disorders without coronary involvement. It is important to reach a definitive diagnosis because MINOCA patients have impaired survival rate compared with age- and sex-matched healthy individuals.3,9–11
Definition and Pathophysiology of MINOCA
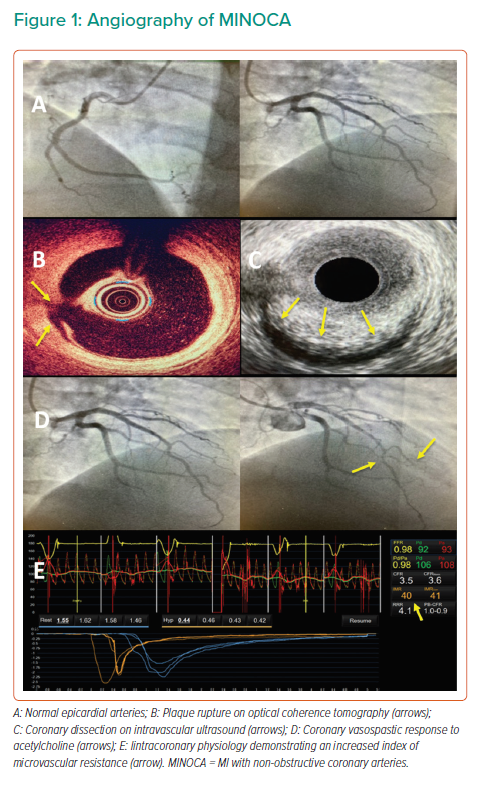
The diagnosis of MINOCA is dependent on the presence of clinical acute MI and the absence of obstructive coronary disease. In a patient presenting with symptoms of ischaemia, cardiac enzyme elevation and echocardiographic or electrocardiographic features suggestive of acute MI, a working diagnosis is made during angiography in the absence of culprit obstructive coronary artery disease (epicardial coronary artery stenosis ≥50%) or an apparent systemic cause for the presentation.12,13 Approximately one-third of patients have been reported to present with suspected STEMI within an emergency setting and the remaining majority as NSTEMI patients undergoing subsequent angiography.14
This working diagnosis then requires further investigation to establish the underlying pathophysiology for the presentation and prevent inadequate or inappropriate therapeutic strategies.
MINOCA disorders can be classified within the fourth universal definition of MI.15 They may meet criteria for type 1 MI, where epicardial coronary artery disorders are diagnosed, or type 2 MI due to endothelial dysfunction or oxygen supply and demand mismatch, or myocardial injury. Examples of underlying diagnoses in patients with a working diagnosis of MINOCA are summarised in Table 1.
Diagnosis and Evaluation of Patients with MINOCA
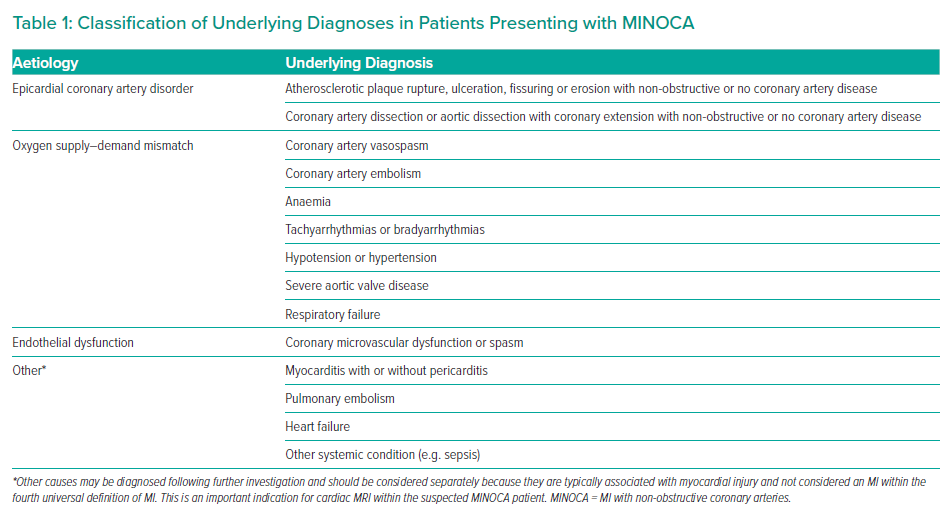
Where a patient meets the criteria for a working diagnosis of MINOCA (universal acute MI criteria, infarct-related epicardial stenosis ≤50%, absence of overt alternative systemic cause) during angiography, then further invasive and adjunctive investigations should be considered at this point (Figures 1 and 2).13 Coronary intravascular ultrasound (IVUS) or optical coherence tomography (OCT) enables the operator to assess for ‘missed’ obstructive disease or dissection in addition to causes of type 1 MINOCA (plaque rupture, erosion, ulceration, intraplaque haemorrhage). Atherosclerotic plaque disruption has been identified using IVUS in approximately 40% of cases of MINOCA.16,17 Reynolds et al. visualised plaque rupture, intraplaque cavity or layered plaque using OCT in 46% of women enrolled in a recent study (STEMI at presentation in 3.5%) and OCT combined with CMR identified the underlying MINOCA diagnosis in 85% of included patients (64% ischaemic aetiology).18 However, while providing insights into MINOCA patients with atherosclerosis, a limitation of that study was that enrolment was limited to 14% (170/1,173) of eligible patients, and so the results may not be representative of all patients with MINOCA.
Further invasive investigations include coronary pressure wire to assess for coronary microvascular dysfunction and vasospasticity. These should be considered once intracoronary imaging has ruled out coronary dissection or plaque disruption or rupture. In order to evaluate for microvascular and vasospastic abnormalities, coronary flow reserve (CFR; abnormal <2.0) should be measured and the index of microcirculatory resistance (IMR; abnormal ≥25) calculated. Fractional flow reserve is not valid in culprit coronary arteries and may be useful for the evaluation of non-culprit coronary artery disease. In the absence of results suggesting microvascular disease (e.g. normal CFR and IMR) and no epicardial stenosis, vasospasticity can be assessed using acetylcholine testing to investigate for epicardial or microvascular vasospasm. Left ventriculography may also be of value in the assessment of other causes, such as takotsubo syndrome, and is routinely performed in many percutaneous coronary intervention (PCI) centres in addition to measurement of left ventricular end-diastolic pressure (LVEDP). Ventriculography may also indicate an epicardial territorial distribution of impaired kinesis implicating a single epicardial artery, compared with a microvascular pattern involving an extended territory of one or more arteries. The upper limit of normal for LVEDP is 10 mmHg, and LVEDP >18 mmHg is associated with an adverse post-MI prognosis.19
Periprocedural laboratory investigations in patients with MINOCA should include relevant biochemical and haematological tests (i.e. serial cardiac troponin measurement, N-terminal pro B-type natriuretic peptide, coagulation screen and haemostasis, D-dimer, full blood count, renal function, electrolytes, glucose and C-reactive protein). If an underlying infection is suspected, serum cultures should be obtained and screening for viral (e.g. SARS-CoV-2 infection) and additional bacterial sources considered.
Following invasive angiography, transthoracic echocardiography should be performed specifically assessing for the presence of regional wall motion abnormalities, embolic sources, pericardial effusion and typical features of takotsubo syndrome. Further echocardiographic assessment for patent foramen ovale and atrial septal defect as embolic sources may be considered using transoesophageal or bubble contrast echocardiography.
Cross-sectional CT should be performed where other causes are suspected (e.g. SARS-CoV-2 infection, pulmonary embolism or aortic dissection). CT coronary angiography is not guideline indicated, but may be of value where intravascular imaging has not been performed during angiography and diagnostic uncertainty remains to assess for intramural haematoma, dissection and the burden coronary plaque disease.
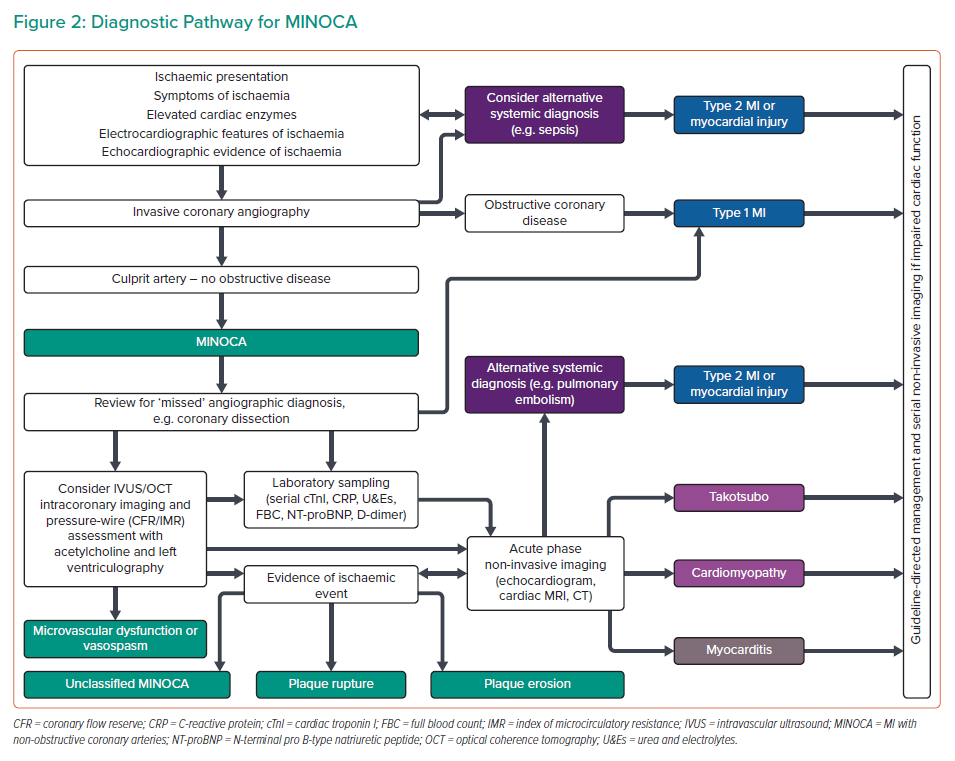
CMR can identify inflammation, oedema and scar and can assess myocardial function by T1- and T2-weighted imaging. CMR is an important diagnostic tool and is guideline recommended in all patients with MINOCA.12 If present on CMR, late gadolinium enhancement localises the site of myocardial damage, and the pattern of distribution suggests the diagnosis (Figure 3). Subendocardial or transmural enhancement is typically of an ischaemic aetiology or hypereosinophilic syndrome. Subepicardial enhancement may be observed in myocarditis, cardiac sarcoid or cardiomyopathy associated with Duchenne muscular dystrophy. Mid-wall enhancement is associated with dilated cardiomyopathy, hypertrophic cardiomyopathy, Duchenne muscular dystrophy, Becker’s muscular dystrophy, Anderson–Fabry disease, sarcoidosis or myocarditis. Finally, global endocardial enhancement is associated with amyloidosis, systemic sclerosis, hypereosinophilic syndrome or Churg–Strauss syndrome, whereas the absence of late gadolinium enhancement may be in keeping with microvascular dysfunction or a non-cardiac cause of the presentation. CMR should be performed as soon as feasible after identification of MINOCA. However, there may be logistical issues with performing CMR in the acute setting (e.g. accessibility of CMR) and it is therefore often performed during the convalescent phase of the illness. This limits the diagnostic yield and certainty of the underlying diagnosis, limiting the potential for acute and appropriate pharmacological intervention.
Therapeutic Strategies for Patients with MINOCA
The treatment of MINOCA requires an individualised approach depending on the underlying diagnosis and may be limited by clinicians’ access to CMR within PCI centres or the use of CMR as a diagnostic adjunct. There remains a paucity of randomised control trial data on treatment in MINOCA, although observational and registry studies have reported lower mortality in MINOCA patients who received renin–angiotensin–aldosterone system (RAAS) inhibitors and statins, including a propensity score-matched analysis of 9,138 patients with MINOCA within the SWEDEHEART registry.20,21 The results indicate long-term beneficial effects of treatment with statins and angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) on outcomes in patients with MINOCA, a trend towards a positive effect of β-blocker treatment and a neutral effect of dual antiplatelet therapy.20,21
MINOCA-BAT (NCT03686696) is a randomised trial of a β-blocker and ACEi or ARB versus placebo involving 3,500 MINOCA patients. The primary outcome of MINOCA-BAT is mortality or readmission due to MI, stroke or heart failure, and the trial is due to complete in 2025. The results of that study may affect future treatment guidelines for patients with MINOCA.22 Mineralocorticoid receptor antagonists (MRA) may have a theoretical role in improving outcomes of MINOCA patients because aldosterone levels immediately after acute MI are associated with all-cause mortality. Aldosterone mediates the downstream effects of RAAS activation, including endothelial dysfunction, inflammation and fibrosis, but, at present, there are no trial data of MRA therapy in MINOCA patients.23
While we wait for trial evidence, it is currently recommended that patients with MINOCA secondary to plaque disruption or with evidence of ischaemic damage on CMR receive dual antiplatelet therapy (12 months followed by lifelong single agent), high-dose statin (including in patients with minimal plaque burden), β-blocker and ACEi or ARB.12 The rationale for this is comparable to that for obstructive coronary artery disease, because thrombosis and/or thromboembolism are thought to be instrumental in the pathogenesis of plaque disruption in MINOCA and statin therapy improves plaque stability even in minimal atherosclerotic disease. Patients with coronary artery dissection should receive aspirin and β-blockers, with additional antiplatelet agents (e.g. clopidogrel) and ACEi/ARB or statins considered.
Coronary embolism and MINOCA may be transient and therefore a diagnostic challenge. Where it is the established diagnosis, treatment of the hypercoagulable state (e.g. diabetic ketoacidosis, heparin-induced thrombocytopenia) in addition to antiplatelet (or anticoagulation where an embolic source is identified), ACE/ARB and statin is appropriate.
MINOCA patients with an underlying diagnosis of epicardial or microvascular vasospasm should receive calcium channel blockers, although nitrates and potassium channel activators may be considered as adjuncts in addition to ACEi/ARB treatment after MI, and statin therapy may be considered if coronary atherosclerosis is identified.
Patients with structural microvascular disease should receive anti-anginal therapy in addition to treatment with ACEi/ARB following MI and statin. Microvascular disease is often under- or untreated, and effective treatment may benefit from a stratified approach with trials for novel therapeutic options awaited.24,25
Treatment for diagnoses of supply–demand mismatch depends on the underlying cause, although additional secondary prevention may be indicated in the presence of mild or non-culprit coronary artery disease.
Outcomes of Patients with MINOCA
MINOCA is frequently collated into a single entity within observational studies, but the prognosis and outcomes of patients depend on the underlying diagnosis for their presentation. Mortality and the incidence of major adverse cardiac events (MACE) for MINOCA patients are reported as comparable with those of patients with obstructive coronary artery disease, and significantly worse than for the general population.3,21
Within the SWEDEHEART registry, approximately one in four patients experience a MACE within 4 years, including death, recurrent MI, hospitalisation with heart failure or ischaemic stroke.26 Although that registry does not use current European Society of Cardiology MINOCA criteria and should therefore be interpreted with caution, a large systematic review and meta-analysis of 1,924 MINOCA patients reported that all-cause mortality at 12 months was 4.7% (SWEDEHEART mortality 2.4% at 6 months).2,14 This reflects an unmet clinical need for effective preventative therapy in this patient group, which is typically younger with fewer comorbidities than patients with obstructive coronary artery disease.14
Risk stratification is challenging in patients with MINOCA where the inciting aetiology is uncertain. However, increased severity of atherosclerosis and elevated serum C-reactive protein are associated with impaired prognosis and are quantifiable during routine assessment.2 Atherosclerotic plaque rupture and related local or systemic inflammation are associated with an increased risk of recurrent events compared with plaques with an intact fibrous cap or lack of objective inflammation (e.g. identified with OCT or IVUS during angiography or subsequently on MRI in addition to serum inflammatory markers).27
These techniques may therefore aid in prognostic stratification, and recent evidence suggests a potential prognostic role for coronary CT angiography with the assessment of pericoronary fat index (pFAI). Higher pFAI values and an increased prevalence of higher-risk non-obstructive intracoronary plaques have been observed in MINOCA patients compared with controls with non-obstructive coronary disease.28
Although there are no studies focused on the effects of MINOCA on quality of life, including persistent ischaemic symptoms and psychosocial parameters, the CorMicA trial demonstrated that, in patients with angina symptoms and/or signs of ischaemia with no obstructive coronary artery disease, diagnostic certainty and appropriate stratification of medical therapy can improve both symptoms of ischaemia and quality-of-life scoring.24 MINOCA-BAT will include a substudy assessing the prevalence of angina pectoris in addition to health-related quality of life, anxiety, depression and psychiatric comorbidities.22
Conclusion
MINOCA is a heterogeneous working diagnosis that requires a multimodal approach to investigation, both during angiography and subsequently with CMR. Identification of the underlying cause is paramount, although, based on observational data, approximately two-thirds of cases may be related to plaque disruption. Although treatment is currently empirical and clinical trials are ongoing, guideline-based stratified therapeutic strategies to improve mortality and MACE will require further large randomised trials.
Clinical Perspective
- MI with non-obstructive coronary arteries (MINOCA) is a heterogeneous working diagnosis requiring further investigation during and after invasive angiography.
- Clinicians should consider the use of intracoronary imaging and coronary physiology testing during angiography to assess for plaque disruption and vasospasticity.
- Cardiac MRI with gadolinium contrast is recommended in all MINOCA patients.
- MINOCA is not benign and has comparable outcomes with acute MI due to obstructive coronary artery disease.
- Treatment of the underlying cause is paramount although, at present, often empirical.
- There is an unmet clinical need for stratified therapy for patients with MINOCA.