Dr Schrage opened his presentation by recapping the use of mechanical circulatory support (MCS) devices to prevent patients from deteriorating into cardiogenic shock (CS) by supporting the patient and providing end organ perfusion. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is one such device increasingly used in cases of severe CS. However, a major drawback associated with VA-ECMO is the increase in left ventricular (LV) afterload, which can result in subsequent LV overload and worsening of the dilatation.1,2 Recent retrospective clinical studies have shown that active LV unloading using a percutaneous transaortic LV assist device, namely Impella, in combination with VA-ECMO reduced LV afterload and was associated with better outcomes and lower mortality.2
Dr Schrage explained that adding the Impella to VA-ECMO, the ECPella configuration, presents an incredible opportunity to optimise LV unloading and facilitate recovery. However, adding a second MCS device also generates additional patient management needs that must be considered. Factors to take into account when managing VA-ECMO and Impella devices simultaneously include:
- balancing the Impella P-level versus ECMO flow: increasing the Impella P-level will allow more active unloading, but may lead to suction events, which can lead to haemolysis;
- assessment of the recovery status of the heart by monitoring the degree of inflow to the LV to monitor when is the time to wean or escalate; and
- ventilation and volume settings of the patient.
Given this, there needs to be continuous monitoring and adjustment of the two devices by balancing the Impella P-level to the LV preload as well as the VA-ECMO flow. However, real-time device management can be challenging while taking care of CS patients; a tool to help automate this process would be immensely helpful.
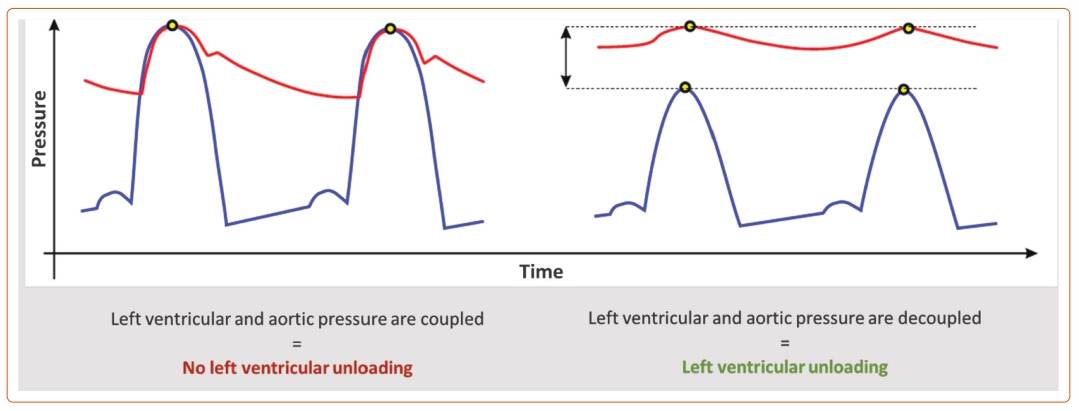
During systole, LV and aortic pressures are coupled: the LV pressure rises and matches the aortic pressure to open the aortic valve and push the blood into the systemic circulation. Impella achieves LV unloading by decoupling the LV pressure from the aortic pressure so that the LV pressure remains below the aortic pressure (Figure 1). This way, the LV can rest and recover while VA-ECMO and Impella support meet the circulatory needs.
Using the degree of LV–aortic pressure decoupling as the metric of LV unloading, a new software algorithm to control Impella pump performance during simultaneous use of VA-ECMO was developed in collaboration with the Abiomed engineering team. The new software tool, named VA-ECMO Mode, is integrated into the Automated Impella Controller (AIC) to allow for automatic control of the Impella P-level to balance against the VA-ECMO flow and avoid suction events, while maximising the degree of LV unloading. This will help lighten the device management burden on the clinical care team and allow for real-time optimisation of the degree of LV unloading to facilitate the patient’s heart recovery. VA-ECMO Mode is currently being tested in a clinical study, with Dr Schrage’s centre being part of it.
Dr Schrage provided a case example of the VA-ECMO mode used in a real-world CS patient with ECPella support. The time course of LV and aortic pressures, aortic pulsatility, the decoupling factor and the Impella pump speed over the 7 days of Impella support was shown. In the first 2 days, placement of VA-ECMO was able to achieve sufficient aortic pressure and mean arterial pressure. The patient, however, exhibited very low pulsatility and almost no native heart function. During this stage, the VA-ECMO Mode algorithm automatically controlled Impella flow and maintained the optimal decoupling factor with little P-level change. Over the next 2 days, as the patient’s native heart function started to recover and pulsatility returned, the VA-ECMO Mode algorithm dynamically adjusted the Impella P-levels to maintain the optimal decoupling and avoid suctioning. This traditionally would be performed manually by the care team. The advantage of the VA-ECMO Mode is that the algorithm can respond immediately to haemodynamic changes, whereas the care team cannot always be present to make immediate changes. In the last 3 days, the patient’s pulsatility stabilised and the care team started weaning the patient off VA-ECMO while the Impella remained in situ to provide ongoing support.
After sharing the successful case of using the VA-ECMO Mode to manage an ECPella patient, Dr Schrage summarised his talk. These clinical experiences support that active LV unloading may improve outcomes for CS patients on VA-ECMO. With opportunities to improve patient outcomes, simultaneous use of two MCS devices also brings about the challenge of proper device management, such as the risk of too much (e.g. suction events, haemolysis) or too little unloading (e.g. non-effective application) unloading. The VA-ECMO Mode automatically decouples LV pressure from aortic pressure and optimises the degree of LV unloading. Dr Schrage closed by highlighting the potential for the VA-ECMO Mode to be used as a guide for device weaning decisions.