In recent years, radial access approaches have gained more attention than traditional femoral access approaches because of better patient satisfaction and safety profiles with earlier mobilisation and shorter hospitalisation durations. Moreover, the use of the radial approach has become the first-line choice for performing percutaneous coronary intervention in acute coronary syndrome (ACS).1,2 Nevertheless, a hostile course of the right subclavian artery (RSA), brachiocephalic artery (BCA) or ascending aorta can be limitations of the right radial access approach.
Difficulties may lead to procedural prolongation and reperfusion delay, which can negatively affect the outcome of ACS patients.3,4 Moreover, other complications have been reported, such as access failure and incomplete examination, in addition to arterial dissection or perforation that may result in mediastinal or retropharyngeal haemorrhage.5–7
Several clinical factors can predict the tortuosity of the RSA or BCA, including older age, female sex, hypertension and a high BMI. However, reports in the current literature have not sufficiently evaluated the radiological predictors of severe tortuosity in the RSA. We hypothesised that chest radiography would add predictive value to the traditional factors in patients selected for coronary angiography with right transradial access (TRA).
Patients and Methods
This prospective study was conducted in the Saudi German Hospital in Cairo, Egypt. The study protocol was approved by the institutional review board in March 2021. The study included all patients who underwent TRA coronary angiography between April 2021 and August 2022. All patients provided written informed consent before the procedure. The following patients were excluded from the study: those post coronary artery bypass surgery, as it is difficult to cannulate the left internal mammary artery with right radial access; those with end-stage renal artery disease on haemodialysis, to avoid damage to arteriovenous shunts; patients in whom it was not possible to achieve a radial artery puncture or those with radial spasm; previously enrolled patients who needed additional coronary angiography; and those who were haemodynamically unstable.
A right radial artery puncture was performed after local subcutaneous lidocaine injection followed by insertion of a 6 Fr radial sheath (Radifocus Introducer II Transradial Kit, Terumo) using a modified Seldinger technique. Next, 5 ml of saline solution containing 5,000 IU heparin was administered through the sidearm of the arterial sheath. For coronary angiography, first, a 0.038 inch guidewire coated with polytetrafluoroethylene and supported with a J-tip fixed core (Merit Medical System) was introduced into the ascending aorta. When the guidewire encountered resistance in the RSA area due to tortuosity, the guidewire was replaced with a 260 cm and more flexible hydrophilic guidewire (Radifocus, Terumo). Then, a diagnostic Tiger II 4 catheter (Radifocus Optitorque, Terumo) was used for coronary angiography. In the case of unavailability, standard JL 3.5 and JR 3.5 6 Fr catheters (Cordis) were used for left and right coronary angiography, respectively. Successful coronary angiography was defined as good visualisation for all coronary arteries with successful cannulation of the left and right arteries in addition to determining the site and severity of any lesion.
In the absence of a consensus definition for the severity of tortuosity in the RSA, BCA or ascending aorta, the difficulty of coronary angiography was graded by the operator according to the Rigatelli et al. classification as follows:8
- Grade 1: no tortuosity or calcification in the RSA or BCA or ascending aorta. Consequently, the diagnostic or guiding catheter could be crossed using a standard non-hydrophilic wire (InQwire, Merit Medical) assisted with deep inspiration, resulting in successful coronary angiography.
- Grade 2: mild tortuosity or calcification of the RSA or ascending aorta. Consequently, the diagnostic or guiding catheter could be crossed with a hydrophilic wire (Soft 0.035 inch Radifocus Guide Wire M, Terumo), resulting in successful coronary angiography after a few manipulations to engage both coronary ostia.
- Grade 3: congenital anomalies or moderate tortuosity or calcification in the RSA or ascending aorta that required a stiff wire (0.035 inch Amplatz Super Stiff, Boston Scientific), and a standard catheter or multiple different or special catheters to engage the coronary ostium and perform successful coronary angiography.
- Grade 4: congenital anomalies or severe tortuosity and/or calcification in the RSA or ascending aorta that prevent the guide catheter from reaching the aortic valve plane or engaging one or both coronary ostia with a stiff wire (hostile subclavian anatomy), thus, requiring another arterial access site to perform successful coronary angiography.
Demographic data – risk factors for coronary artery disease, including smoking, diabetes, hypertension and dyslipidaemia, radiographic data and procedural data were collected for all patients.
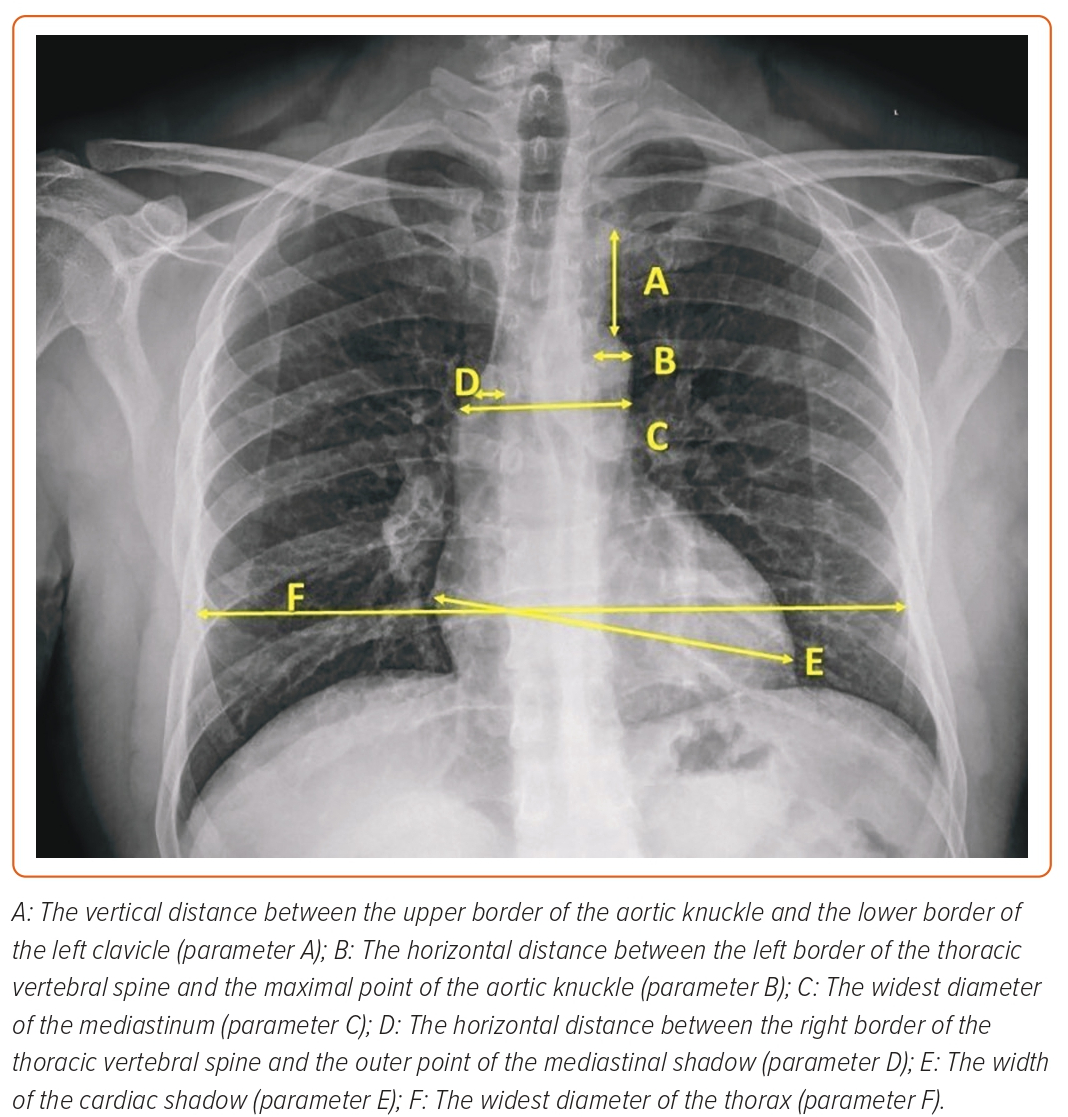
Radiographic data were obtained with a chest radiography posteroanterior view using a digital Philips Dura machine and were analysed by radiologists, who were blinded to angiographic data, using PaxeraHealth software (Figure 1). The radiological data collected were:
- calcification of the aorta and coronary arteries;
- the vertical distance between the upper border of the aortic knuckle and the lower border of the left clavicle (parameter A);
- the horizontal distance between the left border of thoracic vertebral spine and the maximal point of the aortic knuckle (parameter B);
- the widest diameter of the mediastinum (parameter C);
- the horizontal distance between the right border of the thoracic vertebral spine and the outer point of the mediastinal shadow (parameter D);
- the width of the cardiac shadow (parameter E); and
- the widest diameter of the thorax (parameter F; Figure 1).
The following procedural data were collected: fluoroscopy time from insertion of the right radial sheath to complete successful coronary angiography, procedure time from insertion of the right radial sheath to complete successful coronary angiography and radiation dose from the insertion of the right radial sheath to complete successful coronary angiography.
Patients were divided into four groups according to the TRA difficulty grade:
- Group I – patients with grade 1 difficulty.
- Group II – patients with grade 2 difficulty.
- Group III – patients with grade 3 difficulty.
- Group IV – patients with grade 4 difficulty.
Data Management and Analysis
Data were coded and analysed using SPSS, version 25 (IBM Corp.). Normally distributed variables are reported using the mean ± SD and range. The significance of the difference between two means was evaluated using Student’s t-test; the Mann–Whitney Test (U-test) was selected for non-normally distributed variables.
Categorical variables are presented using frequency and percentage, and compared using a χ2 test. In contrast, Fisher’s exact test was used when the expected count was less than 5 in more than 20% of the cells.
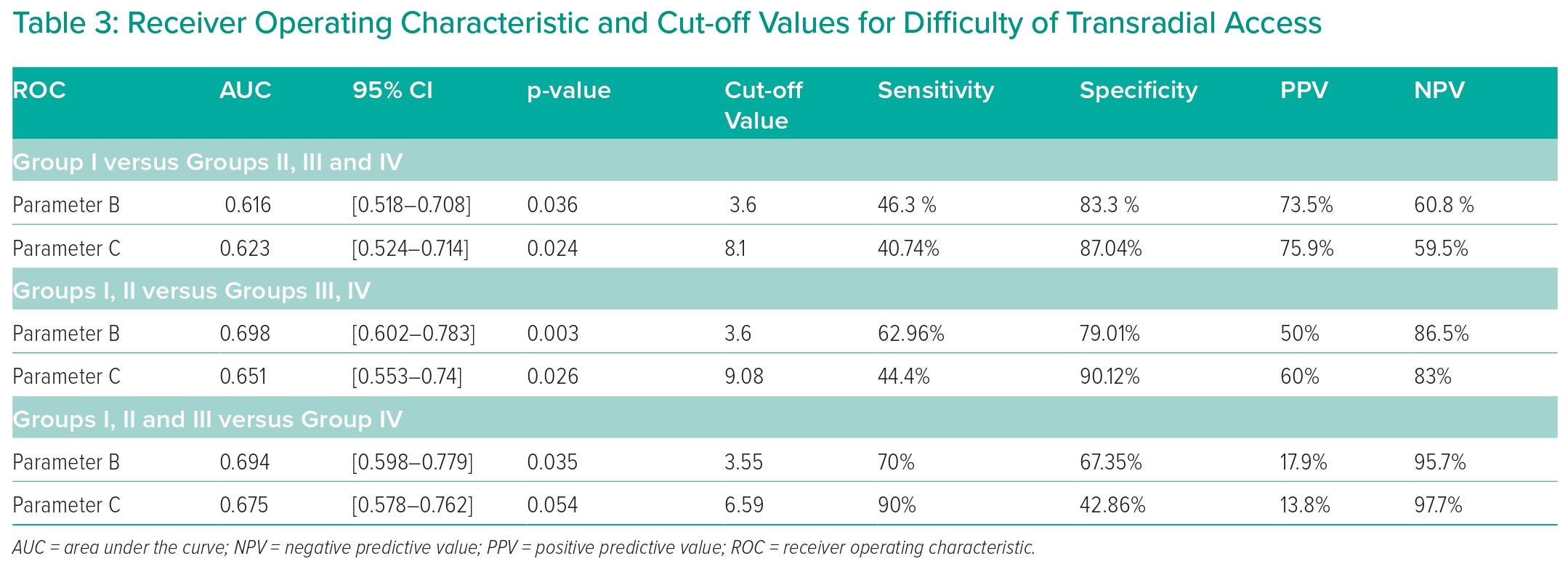
The receiver operating characteristic (ROC) curve was used to evaluate the sensitivity and specificity of the quantitative diagnostic measures of both the radiographic aortic knuckle prominence distance (parameter B) and the widest diameter of the mediastinum (parameter C). The significance level was considered p<0.05.
Results
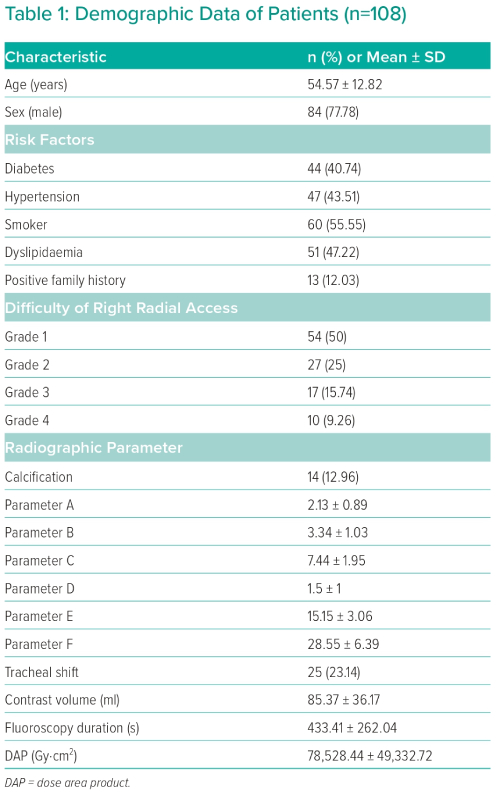
Overall, 127 patients who underwent TRA coronary angiography were included in this study and 19 patients were excluded: one patient because of ill-defined cardiac borders because of lung opacity on the radiographic image, two who refused to enter the study, three who had coronary artery bypass grafting (CABG), two who were unstable haemodynamically, one who was on haemodialysis and 10 with radial spasms. The final sample included 108 patients with a mean age of 54.57 + 12.82 years; 84 were men (77.78%). The demographic data of the patients are shown in Table 1. The patients were divided into four groups based on TRA difficulty. Group I included 54 patients, while Group II, III and IV included 27, 17 and 10 patients, respectively. The rate of TRA failure and crossover to transfemoral access (TFA) was 9.26%.
Comparing Group I with Groups II, III and IV Combined
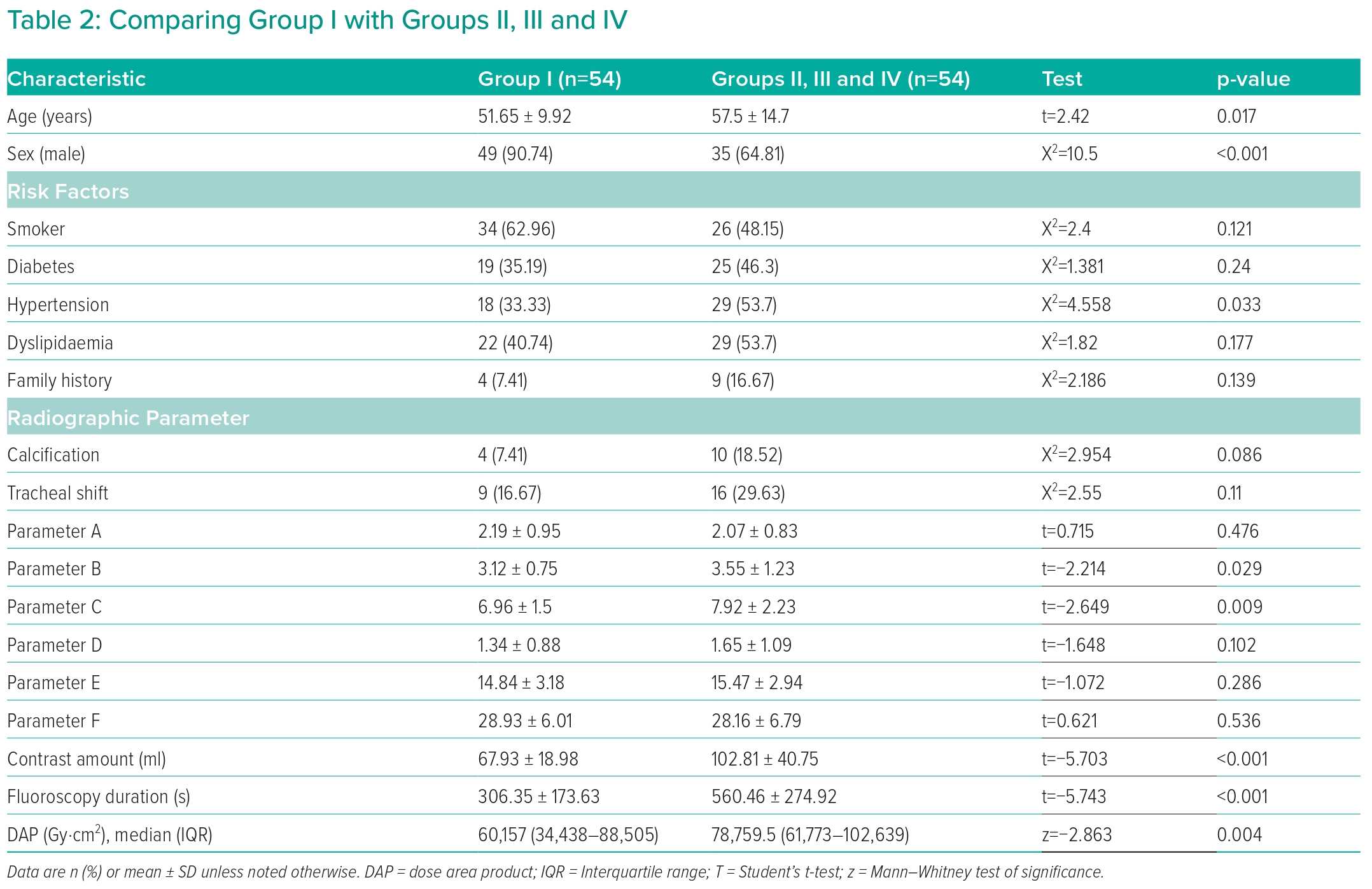
Group I contained patients who had successful coronary angiography without difficulty; this group was compared with other groups. Table 2 presents the difference in baseline characteristics between the groups. The mean age for Group I (51.65 ± 9.92 years) was significantly different from the mean age of Groups II, III and IV (57.50 ± 14.70 years; p=0.017). The number of men in Group I (49; 90.74%) was significantly different from that in Groups II, III and IV (35; 64.81%; p<0.001). There were no other significant differences between the two groups.
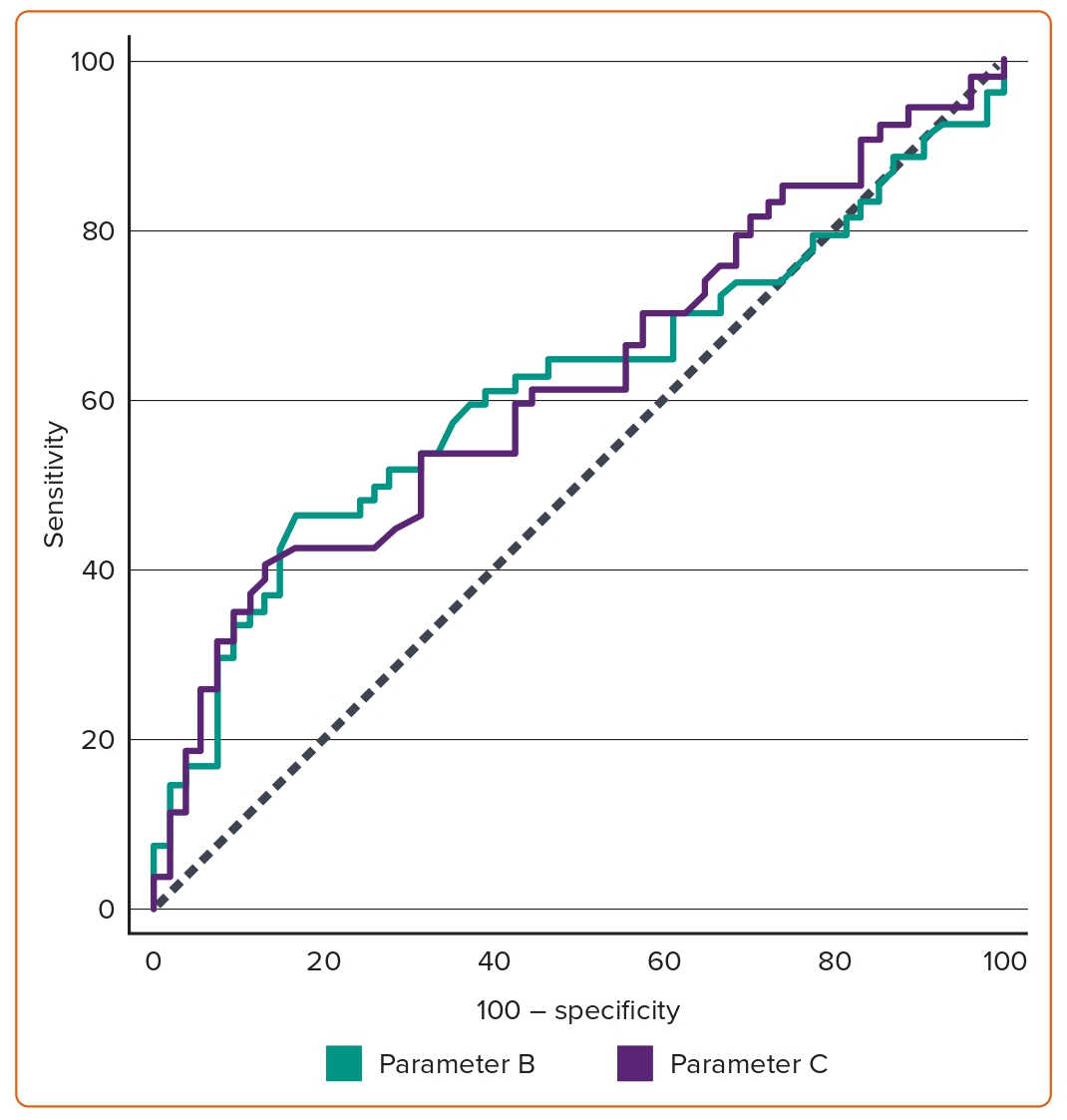
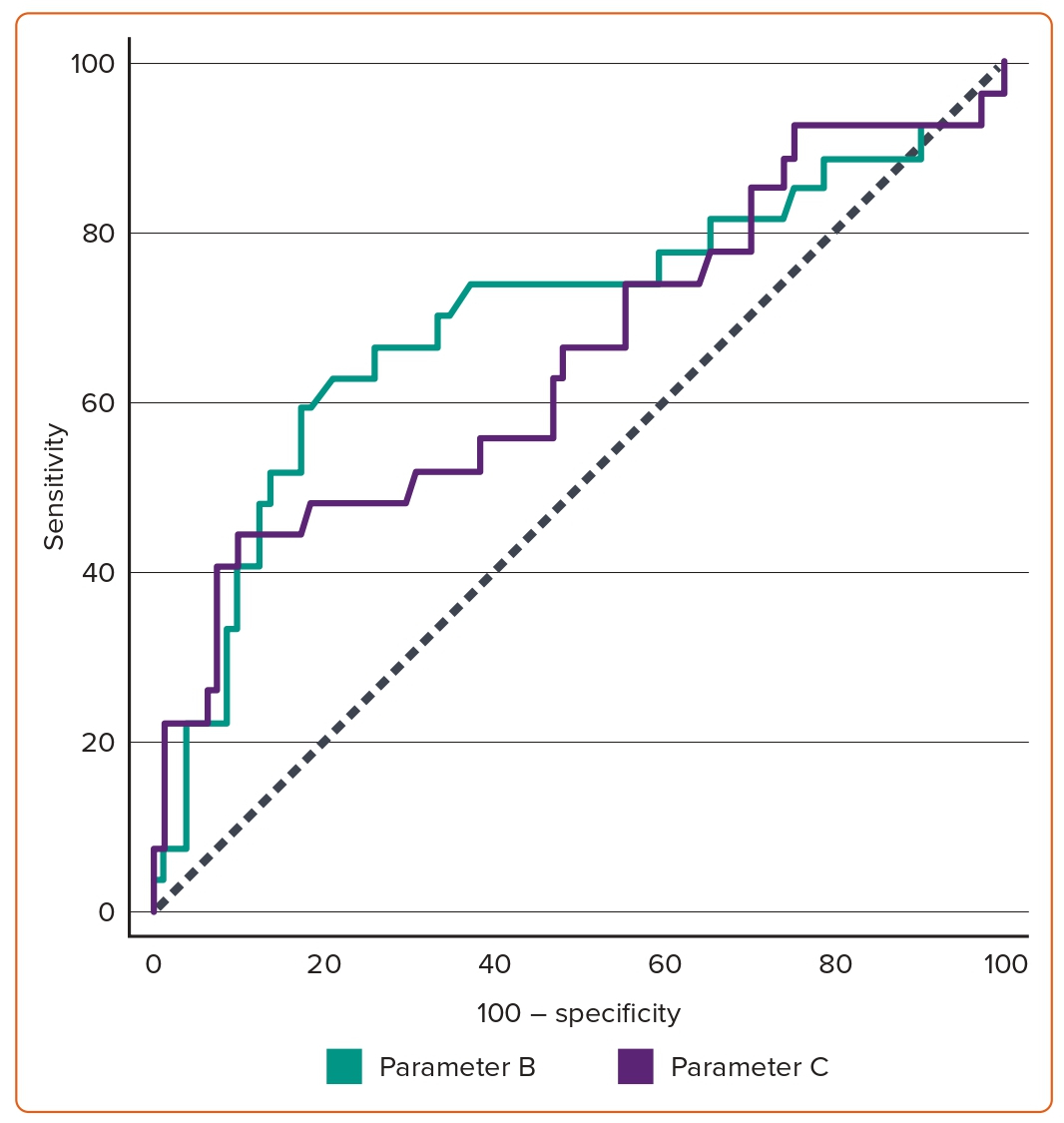
Table 2 also shows the comparison of Group I with Groups II, III and IV regarding radiographic parameters. Parameter B was significantly lower in Group I (Group I, 3.12 ± 0.75; Groups II, III and IV, 3.55 ± 1.23; p=0.029). The same was true for parameter C (Group I, 6.96 ± 1.5; Groups II, III and IV, 7.92 ± 2.23; p=0.009). In Group I, the contrast amount used (67.93 ± 18.98 ml) and fluoroscopy duration (306.35 ± 173.63 seconds) were significantly lower than in Groups II, III and IV (102.81 ± 40.75 ml and 560.46 ± 274.92 seconds, respectively; Table 2). Using the ROC curve, a cut-off value of parameter B above 3.6 demonstrated a sensitivity of 46.3% and specificity of 83.3%, while parameter C had a sensitivity of 40.74% and specificity of 87.04% at a cut-off value above 8.1 (Figure 2 and Table 3).
Comparing Groups I and II Combined with Groups III and IV Combined
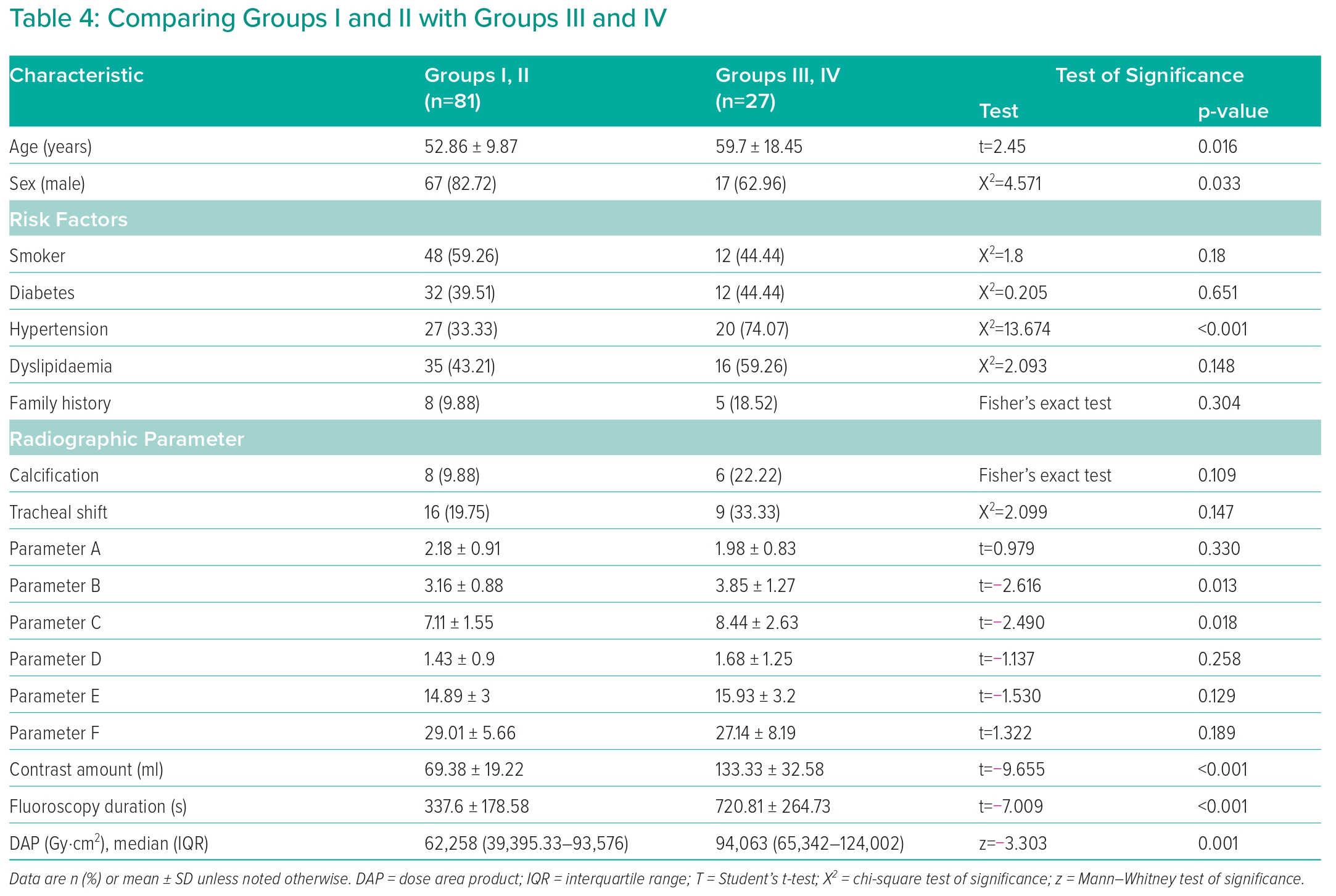
Patients were divided according to TRA difficulty into simple and difficult; the simple group included Groups I and II, while the difficult group included Groups III and IV. Table 4 shows the difference in baseline characteristics between the groups. The mean age of Groups I and II (52.86 ± 9.87 years) was significantly lower than that of Groups III and IV (59.7 ± 18.45 years; p=0.016). The number of men in Groups I and II (67, 82.72%) was significantly higher than in Groups III and IV (17, 62.96%; p=0.033). There were no other significant differences between the two groups.
Table 4 also shows the comparison between Groups I and II and Groups III and IV regarding radiographic parameters. Parameter B was significantly lower in Groups I and II (3.16 ± 0.88) than in Groups III and IV (3.85 ± 1.27; p=0.013). The same was true for parameter C (Groups I and II, 7.11 ± 1.55; Groups III and IV, 8.44 ± 2.63; p=0.018).
In Groups I and II, the contrast amount used (69.38 ± 19.22 ml) and fluoroscopy duration (337.6 ± 178.58 seconds) were significantly lower than in Groups III and IV (133.33 ± 32.58 ml and 720.81 ± 264.73 seconds, respectively; Table 4). According to the ROC curve, a cut-off value of parameter B above 3.6 demonstrated a sensitivity of 62.96% and a specificity of 79.01%. In comparison, parameter C had a sensitivity of 44.4% and specificity of 90.12% at a cut-off value above 9.08 (Figure 3 and Table 3).
Comparing Groups I, II and III Combined with Group IV
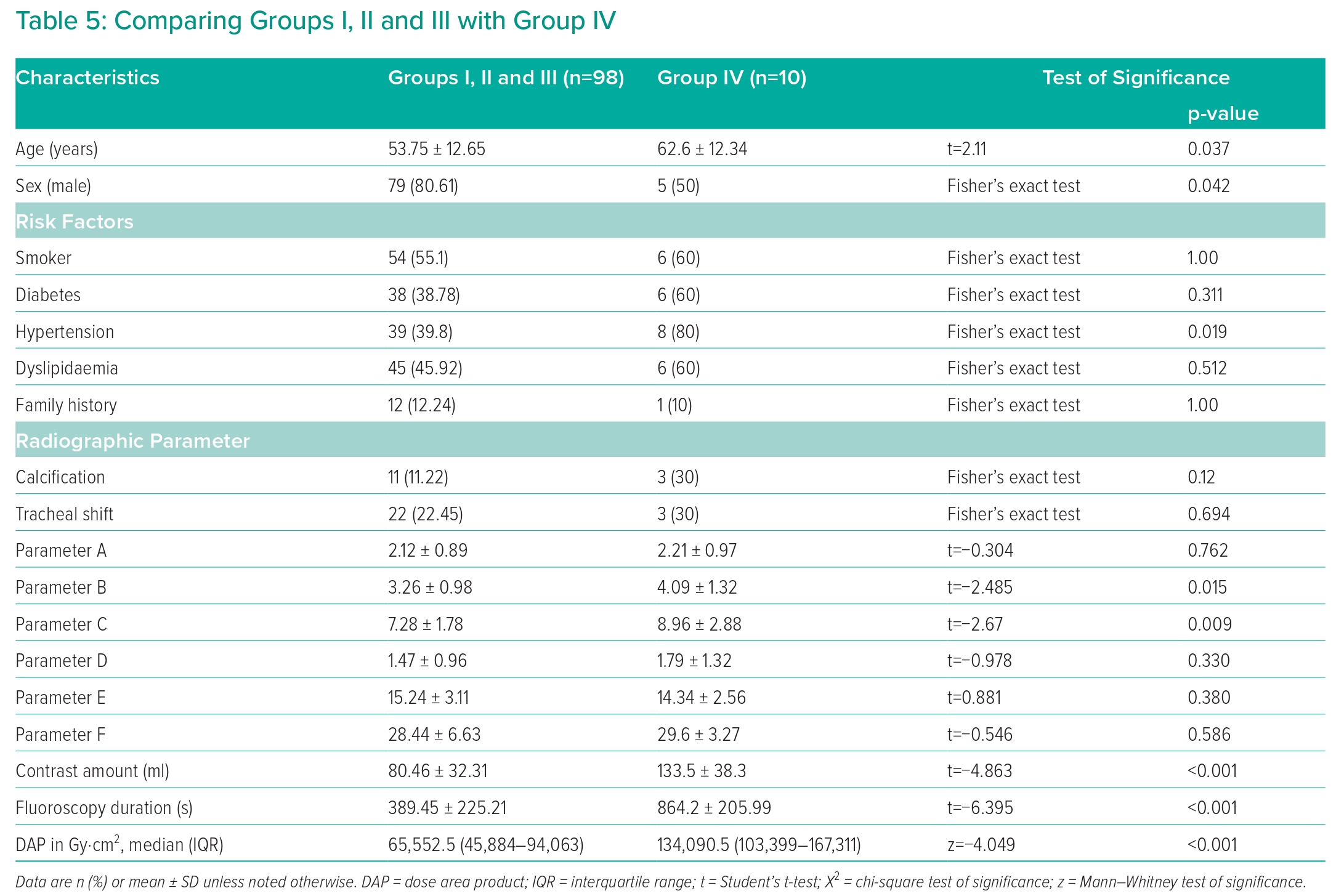
Group IV, where patients had TRA failure, was compared with other groups in which patients had successful TRA coronary angiography with various difficulty degrees, including Groups I, II and III. Table 5 shows the significance of the difference in baseline characteristics between the groups. The mean age for Groups I, II and III (53.75 ± 12.65 years) was significantly lower than the mean age of Group IV (62.6 ± 12.34 years; p=0.037). The number of males in Groups I, II and III (79, 80.61%) was significantly different from that of Group IV (five, 50%; p=0.042). There were no other significant differences between the two groups.
Table 5 also shows Group IV compared with Groups I, II and III combined regarding the radiographic parameters. Parameter B was significantly lower in Groups I, II and III (3.26 ± 0.98) than in Group IV (4.09 ± 1.32; p=0.015). The same was true for parameter C, where it was significantly lower in Groups I and II and III (7.28 ± 1.78) than in Group IV (8.96 ± 2.88; p=0.009).
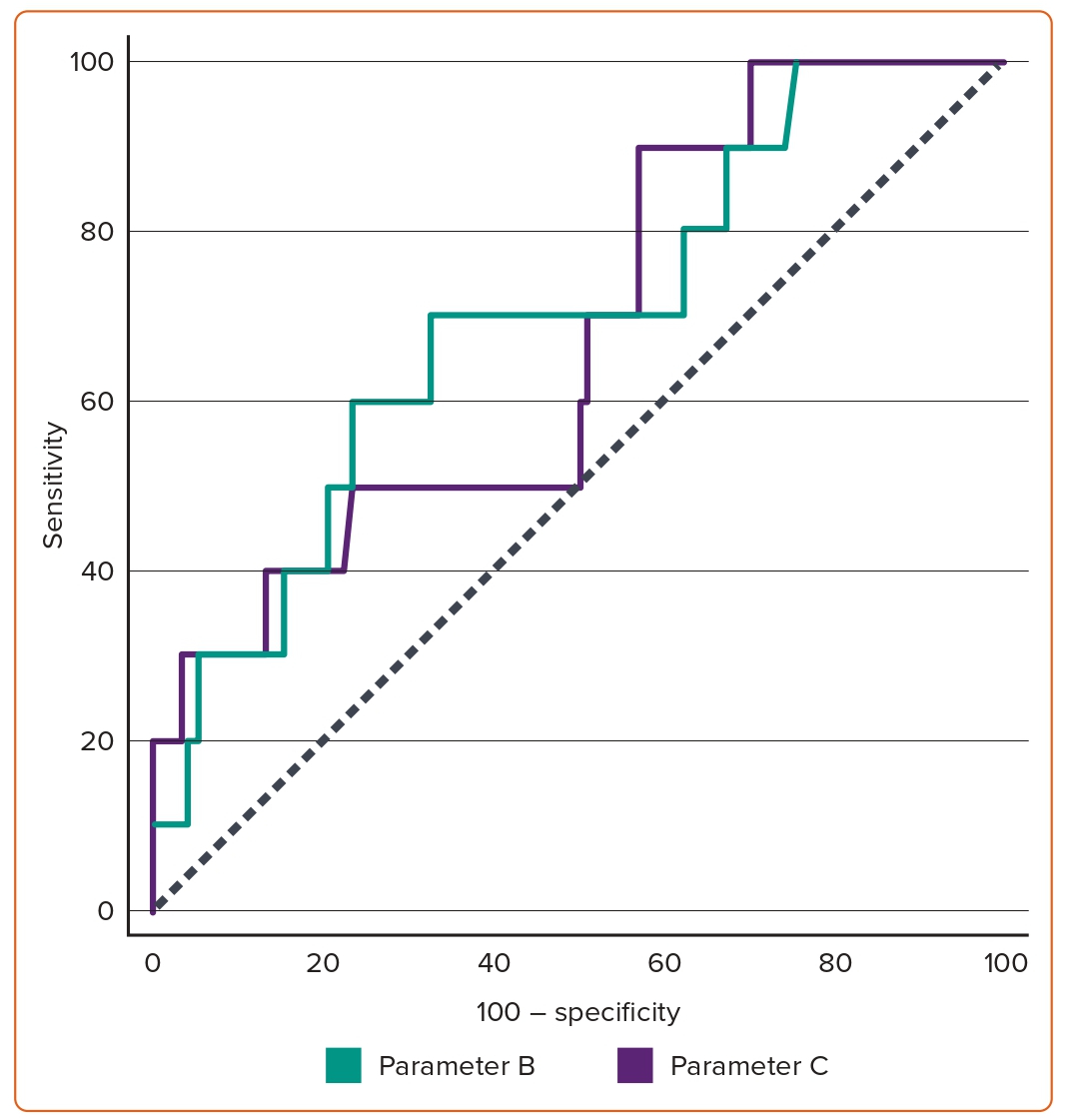
In Group IV, the contrast amount used (133.5 ± 38.3 ml) and fluoroscopy duration (864.2 ± 205.99 seconds) were significantly higher than in Groups I, II and III combined (80.46 ± 32.31 ml and 389.45 ± 225.21 seconds, respectively; Table 5). According to the ROC curve, a cut-off value of parameter B above 3.55 demonstrated a sensitivity of 70.00% and a specificity of 67.35%. In comparison, parameter C had a sensitivity of 90% and specificity of 42.86% at a cut-off value above 6.59 (Figure 4 and Table 3).
Discussion
Determination of vascular site access for coronary angiography is crucial for patient safety and clinical outcomes as it is a significant predictor of 1-year mortality.6,7 TRA is increasingly preferred and adopted worldwide. It also offers many benefits, including fewer bleeding and vascular complications, early ambulation, shorter hospital stays, lower healthcare costs and improved prognosis of patients compared with TFA.1,9–14 However, it has some limitations and complications, as the primary concern with TRA is relatively high failure and crossover rates of up to 11%, which can affect patient care.15–18 TRA failure requires TFA crossover, which is associated with the potential complications of two different puncture sites. Additionally, it can delay coronary intervention in emergencies, where every minute is essential for myocardial survival.19
There are various causes for TRA failure, including anatomical, procedural and pathophysiological factors. Anatomical factors are the major contributor, as they are associated with a higher rate of TRA failure.7,9,17,18 Subclavian tortuosity and unfavourable anatomy of the aortic root are important anatomical factors (6–10% of patients undergoing a transradial approach) that should be considered during a right radial approach.9,15 These factors hinder advancing the guide catheter to the ascending aorta, causing inadequate coronary cannulation or lack of adequate guide catheter backup support. Early recognition or prediction of these anomalies can lead to prompt solutions.15
In our study, the rate of failure of TRA and crossover was 9.26%, similar to rates of failure at 11% documented in the literature, presenting significant challenges for adequate coronary angiography.15–18,20 The high rate of failure and crossover in our study was probably due to the exclusion of patients with radial spasms, which can be overcome using vasodilators and additional manoeuvres as usage of balloon-assisted tracking to overcome difficult radial anatomy. Moreover, further factors could be involved, such as the small sample size in our study. On the other hand, previous studies were not randomised and the choice of puncture site was according to the operator.15–18,20
Clinical Predictors
The present study showed that the clinical predictors of a tortuous RSA were female sex, older age and hypertension. These findings align with the risk factors reported in previous studies, including female sex, multivessel disease, prior CABG, low body weight, age >75 years and short stature.16,17,21 Complex vessel anatomies are another risk factor, which includes subclavian tortuosity, small size, small aortic root and short ascending aorta.22 Although Hu et al. found unsuccessful radial artery punctures in 34 patients (30%), they reported that female sex and age >75 years were independent risk factors of TRA failure in the Chaoshan area. Similarly, our study demonstrated that female sex and advanced age are independent predictors.8
In a study by Sciahbasi et al., being aged ≥70 years was an independent predictor of RSA tortuosity.23 Similarly, a retrospective analysis by Cha et al. identified similar risk factors such as advanced age, female sex and history of systemic hypertension. However, they added non-smoker status, shorter stature and a high BMI to the list.20 Our study showed insignificant results regarding smoking; however, our study did not measure height and BMI. Similar to our study, Dehghani et al. determined that the primary predictors of failure were age ≥75 years, female sex, short stature and history of CABG, except that we excluded CABG patients.17
Tahir et al. used CHA2DS2-VASc scores to predict TRA failure that required crossover to TFA, as this score shares many of the same risk factors between TRA failure and the risk of embolic stroke in patients with AF.15 They found that the higher the score, the higher the rate of TRA failure, with the highest rate at a score of 8 or higher. However, this study did not include patients with aberrant right subclavian arteries (arteria lusoria) or anomalous coronary arteries.15
Radiologic Factors
This study used several radiographic measurements to predict RSA tortuosity. The projection of the aortic knuckle from the left border of the spine and the width of the mediastinum are the two parameters that were statistically significant in predicting the difficulty of a coronary angiography procedure caused by RSA tortuosity. This result conforms with the findings reported by Wahab et al.24 They found a prominent aortic knuckle on the chest roentgenograms of 30 people with Ehlers-Danlos syndrome who had an elongated aortic arch and tortuous BCAs.
Case et al. used the maximum distance of thoracic aorta curvature, defined as the distance from the middle of the patient’s spine to the furthest point reached by the catheter in the thoracic aorta. They found that the operator should use a left coronary system-specific JL catheter if the distance was equal to or exceeded 1 cm. Additionally, they identified a sign formed by the large convex curve of the aorta, followed by a small concave curve resembling an ‘elephant head’.25
Burzotta et al. showed the presence of anatomic vascular variants between the wrist and the aorta affected the success rate of TRA. They used an angiogram to classify variants using a simple, 10-item ABC classification.26 The conclusion was that appropriate recognition of these variants is pivotal for 84.4% of the procedural success rate.27 On the other hand, Nishizaki et al. used chest radiography (posteroanterior view) to predict severe tortuosity of the RSA. They evaluated the cardiothoracic ratio, aortic arch calcification and prominently projected aortic arch. The distance from the neck of the aortic arch to the left edge of the aortic arch was defined as 10 mm. Consequently, the results showed that the presence of a prominently projected aortic arch was a useful predictor.28
Although Christensen et al. showed that a tortuous innominate artery leads to a slight widening of the right upper mediastinum, it frequently buckles to the right, simulating a lung mass tumour and widening of the right upper mediastinum. This can cause prominence to a right apical with a characteristic combination of a poorly defined upper margin and a crisply defined lower margin.29
In this study, comparisons between different groups showed that the increased difficulty of TRA was associated with increased sensitivity and specificity of the cut-off value of both parameters B and C. An exception was the comparisons of Group IV to other Groups, which can be explained by the small sample size of Group IV.
Limitations
Although this study was a blinded prospective study, it has some limitations, including the small sample size and single-centre design. Further larger, multicentre studies are required to validate the results.
Conclusion
The clinical predictors of right TRA failure for coronary angiography included older age, female sex and hypertension. Projection of the aortic knuckle and width of the mediastinum were identified as radiographic parameters that add prediction value to clinical predictors in this study. 
Clinical Perspective
- Right radial access failure and the need for crossover are associated with higher complications and delayed revascularisation.
- Projection of the aortic knuckle and width of the mediastinum are radiographic parameters that add prediction value to clinical predictors.
- Prominent aortic knuckle (3.55 cm) has a sensitivity of 70.00% and specificity of 67.35% for the prediction of transradial access failure; mediastinal width 6.59 cm has a sensitivity of 90% and specificity of 42.86%.
- Radiographic parameters can be validated using angiographic fluoroscopy, which can help in choosing access site for coronary angiography and subsequently reduce complications of access failure and crossover.