AF is the most common cardiac arrhythmia worldwide. The estimated prevalence among adults is 2–4%, and the lifetime risk of developing AF is one in three individuals.1–3 AF is associated with stroke, increasing the risk four- to five-fold while accounting for 20–30% of ischaemic strokes.4,5 In addition, embolic strokes associated with AF are usually severe, with permanent disability and higher mortality.5,6 To lower the risk of stroke in AF patients, the majority are treated with oral anticoagulant (OAC) therapy.7–9
The Challenge of AF and Percutaneous Coronary Intervention
AF and coronary artery disease (CAD) are common and are, therefore, frequent comorbidities. A pooled analysis of data including 8 million patients from 109 studies reporting data in newly diagnosed acute coronary syndrome (ACS) showed the prevalence rate of pre-existing AF was 5.8% and 7.3% for newly diagnosed AF in ACS patients.10 It is estimated that 5.4% of all ACS events are associated with AF and there is a 60–77% risk of developing new AF.11,12 Furthermore, patients with AF are estimated to have a 10–15% chance of proceeding to percutaneous coronary intervention (PCI).13 Clearly, these data highlight the frequency of the challenge associated with the requirement for adequate post-PCI antiplatelet therapy in AF patients who require indefinite formal anticoagulation. What constitutes adequate antiplatelet therapy in this context is a complex question.
Patients with AF and ACS are historically less likely to receive appropriate antithrombotic therapy and therefore are more likely to experience adverse outcomes when compared to ACS patients without AF.14,15 For example, in a survey of 755 patients with AF and ACS, only 22% of patients were prescribed anticoagulation.15 In the GRACE registry of 59,032 patients admitted with ACS, nearly 7,500 patients had either pre-existing AF or newly developed AF during hospital admission. There was a higher rate of hospital death in patients with new-onset (14.5%) and pre-existing (8.9%) AF compared to 1.2% in those without AF.16
Patients with AF who have undergone recent PCI are conventionally put on both oral anticoagulation and dual antiplatelet therapy (DAPT). However, this triple therapy regimen is strongly associated with an increased bleeding risk. In a Danish cohort study of 82,854 patients on combination treatment with warfarin, aspirin or clopidogrel (or both), there was a threefold higher risk of fatal and non-fatal bleeding with warfarin and clopidogrel therapy (13.9% per patient year) and triple therapy (15.7% per patient year) compared to warfarin monotherapy at a mean follow-up of 3.3 years.17
Furthermore, bleeding is associated with an increase in mortality. For example, among 3,345 (92.9%) of 3,602 patients in the HORIZONS-AMI trial who underwent primary PCI for ST-elevation MI (STEMI), 231 (6.9%) developed major bleeding in hospital.18 At 3-year follow-up, patients who had in-hospital major bleeding had higher mortality (24.6% versus 5.4%; p<0.0001) and major adverse cardiac events (MACE; 40.3% versus 20.5%; p<0.0001) in comparison to patients without major bleeding.18 Recently published trials strongly suggest efficacy from an ischaemia point of view, but with a lower bleeding risk, using a strategy of single antiplatelet in combination with OAC – preferably direct oral anticoagulant (DOAC) – when used within the first year after PCI in AF patients.18–21 Combining DAPT and oral anticoagulants, also called ‘triple therapy’, significantly increases all-cause hospitalisation in comparison to dual therapy. Using data from the ORBIT-AF registry, in which 1,827 patients with CAD were randomised according to treatment with triple or dual antithrombotic therapy (90% aspirin, 10% clopidogrel) or two antiplatelet drugs and no anticoagulant, it was noted that patients treated with triple therapy were hospitalised for all causes (including cardiovascular) more often than patients on dual therapy (79 in 100 patient years and 47 in 100 patient years respectively, p<0.0001). However, no differences in stroke, major bleeding or death were seen among the two different antithrombotic therapy strategies.19 The WOEST trial studied the use of clopidogrel and oral anticoagulation compared with triple antithrombotic therapy (aspirin, clopidogrel and OAC) in patients who underwent PCI and found that patients receiving dual antithrombotic therapy had similar ischaemic outcomes with less major bleeding compared with the patients receiving triple antithrombotic therapy.20
In a systematic review of four trials (PIONEER AF-PCI, ENTRUST-AF PCI, AUGUSTUS and REDUAL PCI) involving 7,953 patients comparing dual therapy (P2Y12 inhibitor + discontinuation and OAC) and triple therapy (DAPT + vitamin K antagonists [VKA]) in patients with AF undergoing PCI, with median follow up of 1 year, DOAC-based dual therapy resulted in a lower rate of bleeding events compared with VKA-based triple therapy.21–25
Similarly, in the WOEST trial bleeding episodes were seen in 19.4% of patients receiving double therapy (VKA plus clopidogrel) versus 44.4% receiving triple therapy (aspirin, clopidogrel and VKA; p<0.0001).20 The AUGUST trial compared aspirin with placebo in the patients taking the OAC and P2Y12 inhibitor. Major or clinically relevant non-major bleeding was higher: in >12% in the group receiving aspirin compared to 9% in the placebo group (p<0.001).24
The Clinical Dilemma Summarised
The combination of a coronary stent plus AF introduces an important and common management dilemma that centres on the balance between ischaemic/thromboembolic risk versus the risk of bleeding. Several logical questions arise from this clinical challenge. First, does single or dual antiplatelet therapy offer protection against the intracardiac clot that occurs because of AF? Second, do OAC agents (warfarin or DOACs) have antiplatelet activity and, if so, how potent is it? Third, do OACs reduce the risk of platelet-mediated acute MI and stent thrombosis events, regardless of the biological mechanism? Finally, what balance of ischaemic/thromboembolic events versus bleeding events is clinically acceptable on the assumption that we cannot eliminate either and they are inversely related?
What Do International Guidelines Recommend?
Major societies, including the European Society of Cardiology (ESC), American College of Cardiology/American Heart Association (ACC/AHA) and the Heart Rhythm Society (HRS), have all produced recommendations relating to pharmacological treatment for both PCI and AF patients, as well as those with both.
All guidelines recommend aspirin and a P2Y12 inhibitor together for a certain period after PCI for patients without AF, followed by aspirin alone in the long term. The recommended duration of DAPT varies according to clinical presentation, such as elective or ACS, and bleeding risk. For patients with AF without coronary stents, guidelines recommend formal anticoagulation, generally VKA or novel oral anticoagulation (NOAC) for life if the estimated thromboembolic risk is above a certain threshold, such as for those with a CHA2DS2−VASc score of 1 or more.7,8 For patients who have had PCI and have concomitant AF, guidelines generally recommend an initial period of OAC and antiplatelet therapy in combination. For example, ESC AF guidelines recommend aspirin for 1 week and dual therapy for up to 12 months (preferably DOAC and clopidogrel) as level I evidence, and level II for triple therapy (aspirin, clopidogrel and DOAC) for up to 1 month then dual therapy (DOAC + clopidogrel) in AF patients presenting with ACS where stent thrombosis risk outweighs bleeding risk.7 Similarly, the ACC 2020 expert consensus statement advocates that aspirin be continued for the duration of hospitalisation after PCI and discontinued upon discharge or at 30 days in those patients who are at high thrombotic and low bleeding risk, with dual therapy to continue to 12 months.26
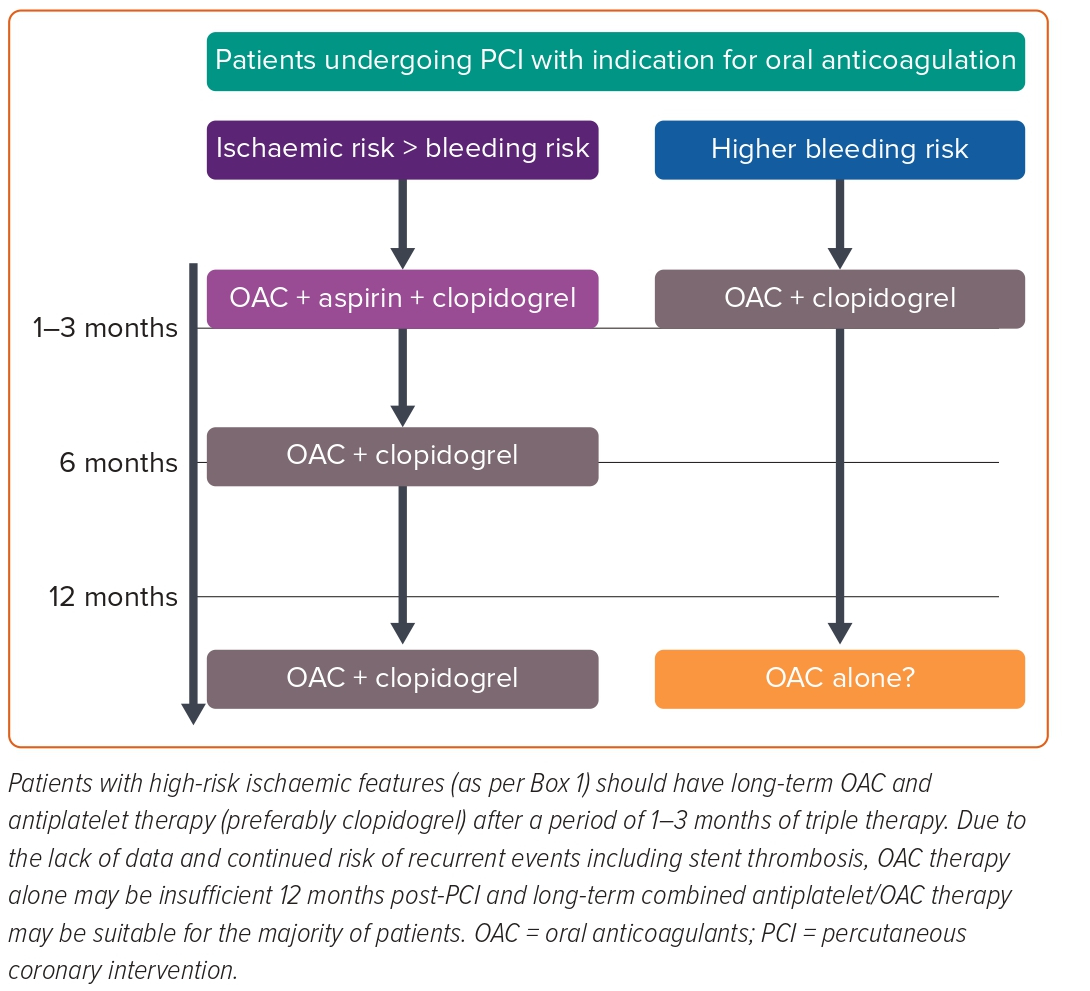
The most recent ESC AF guideline and the ACC expert statements advise discontinuing antiplatelets to use OAC alone after 12 months of PCI.7,26 The most contentious issue – and the focus of this review – is the recommendation that in such patients there is no requirement for antiplatelet therapy at all after 12 months and they should be on OAC alone beyond that time point.26–28
The Main Point of Contention: Is it Safe for Patients with AF to Drop All Antiplatelet Therapy After 12 Months Post PCI?
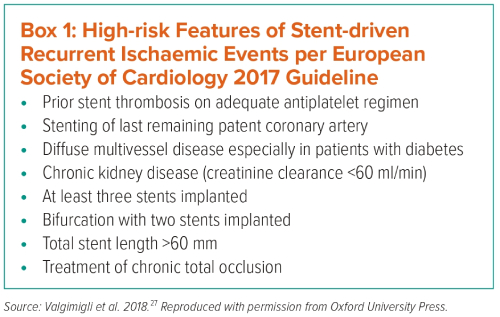
There are several reasons why recommending that OAC therapy alone is sufficient for the AF patient with a stent after 12 months is contentious. First, the commonest time for stent thrombosis to occur is beyond 12 months after implantation – known as very late stent thrombosis (VLST).29,30 Second, there is an attritional rate of stent thrombosis with modern drug-eluting stents (DES) of about 0.5% per annum.31 Third, stent thrombosis is unequivocally a consequence of platelet-mediated thrombus, so that the presence of an antiplatelet agent or an agent with antiplatelet properties at least to inhibit this biological process seems logical. Thus, the question arises: are OACs – either warfarin or NOACs – agents with adequate antiplatelet activity? Finally, if this strategy – which is based on expert consensus – turns out to be flawed, can we expect a steady increase in the rate of attritional VLST over the next few years?
Given all these concerns, we should examine how the guidelines have come to make this recommendation, the evidence upon which this conclusion is made and the ongoing concerns that make many interventionalists unconvinced that this is a dominant strategy.
The Role of Platelets in Preventing Acute MI and Stent Thrombosis
The hallmark of CAD is the build-up of atherosclerotic plaque with the deposition of subendothelial lipid-rich macrophages called foam cells. When there is a breakdown of endothelium, for example with a vascular insult, endothelial cells respond by secreting chemokines that attract white cells to the injured area. There is also a migration of monocytes to the subendothelial space aided by cell surface adhesion molecules released by the endothelium with a subsequent take-up of low-density lipoprotein, resulting in foam cell formation. The slow progression of this subendothelial fat streak with the aid of T-cell cytokines and growth factors leads to fibroproliferation of the medial smooth muscle layer.32
Some plaques can rupture or erode and then expose/release subendothelial matrix, including collagen and von Willebrand factor, which bind to platelet receptors resulting in the adhesion, activation and aggregation of platelets and thrombosis. This thrombosis could cause partial or total occlusion of the lumen and result in the development of ACS.32,33 Platelet activation and thrombus formation are enhanced by three major clinically relevant pathways: adenosine diphosphate (ADP)-P2Y12, cyclo-oxygenase-1 (COX-1) and thrombin.
The specific interaction of ligands and molecules of platelet receptors leads to the activation of platelets and the release of dense platelet granules. ADP is released, which amplifies platelet activation and aggregation. ADP activates P2Y12, which maintains platelet stability. Further, as a result of the effect of thromboxane synthase on prostaglandin H2, thromboxane A2 is produced, which has similar effects on platelet amplification and aggregation.34,35 Concurrent with ADP-P2Y12 activation, the coagulation pathway cascade is activated and, as a result, factor Xa and factor Va lead to thrombin formation/activation. Thrombin is the key enzyme that converts fibrinogen into fibrin. In addition, thrombin activates protease-1-activated receptor (PAR-1) and PAR-4 on platelets, which enhances platelet aggregation.35,36
Mechanism of Action of Aspirin and P2Y12 Inhibitors
Aspirin inhibits the activity of cyclooxygenase, an enzyme responsible for the production of thromboxane A2. Thromboxane is a key component of one of the major pathways for platelet activation and subsequent aggregation. Aspirin also reduces thrombin generation with subsequent attenuation of factor XIII activation.37
The interaction of ADP with the platelet P2Y12 receptor is the essential part in the platelet activation process. Activation of the glycoprotein IIb/IIIa receptor results in enhanced platelet degranulation and thromboxane production and prolonged platelet aggregation. The P2Y12 receptor is the main receptor in the activation of ADP-mediated stimulation of glycoprotein IIb/IIIa receptor activation.38 Both thienopyridines (clopidogrel and prasugrel) and non-thienopyridine drugs (ticagrelor) act by inhibiting the platelet P2Y12 receptor.39
Clinical Evidence for a Reduction of MI and Stent Thrombosis in Various Populations Using Antiplatelet Drugs
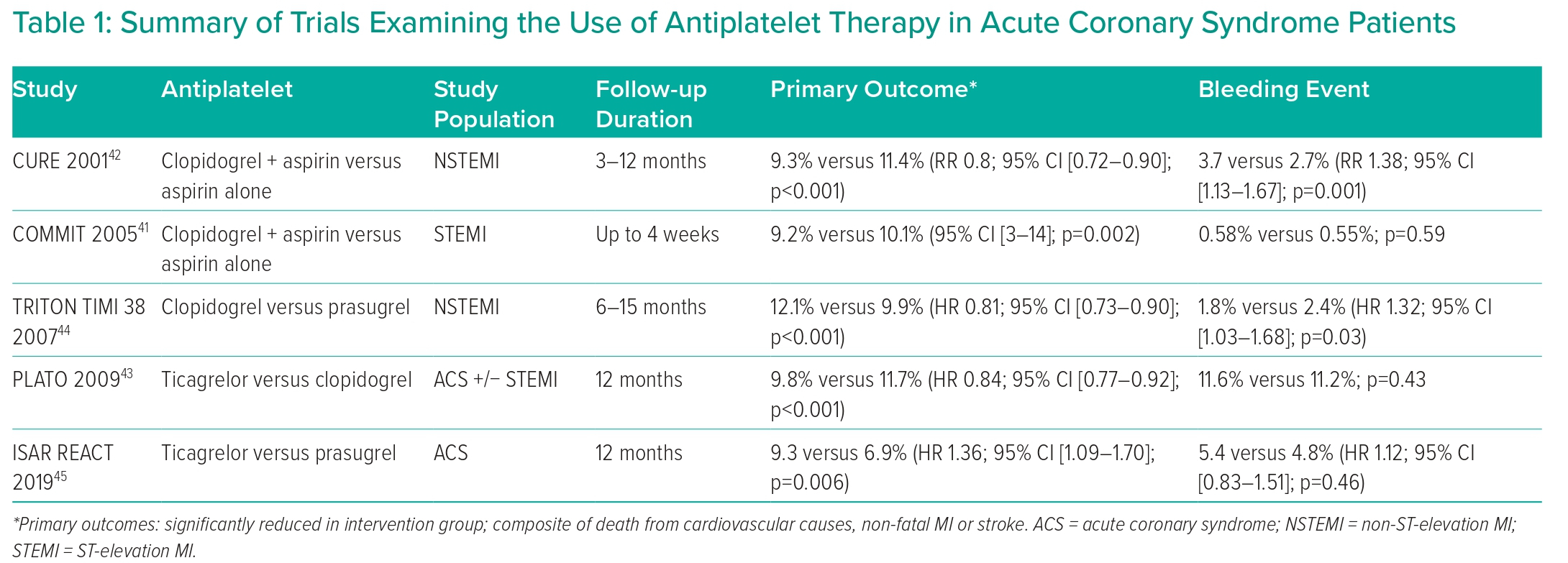
Antithrombotic therapy has been established as a key intervention to prevent stent thrombosis after stent placement.40 Based on more than 35 randomised clinical trials, including more than 225,000 patients, DAPT is among the most intensively investigated treatment option in the field of cardiovascular medicine (Table 1).27
Aspirin has traditionally been the cornerstone of antiplatelet therapy in patients with CAD. The ESC guidelines give a class I recommendation for the use of aspirin in ACS patients.27 Both this guideline and ACC/AHA 2016 ACS guideline give a class I indication for the use of DAPT for up to 12 months after DES implantation for ACS.27,28 Following DAPT, lifelong single antiplatelet therapy (usually with aspirin) is recommended, due to the attritional risks of stent thrombosis and recurrent MI.27,28
The choice of antiplatelet therapy and recommendations about dual and single therapy is dynamic based on accumulating evidence from clinical trials. The evidence strongly suggests that there is clinical benefit for ACS patients adding a P2Y12 inhibitor to aspirin therapy alone.
For example, COMMIT studied the effect of adding clopidogrel to aspirin in STEMI patients, randomising 45,852 patients on aspirin to clopidogrel 75 mg daily (n=22,961) versus placebo (n=22,891). There was significantly lower mortality in the clopidogrel group (7.5% versus 8.1%; p=0.03), as well as a lower rate of a composite of death, reinfarction or stroke (9.2% versus 10.1%; p=0.002).41
A similar outcome benefit from adding clopidogrel to aspirin therapy was seen in NSTEMI patients in the CURE trial. This trial randomised 12,562 patients who had presented within 24 hours after the onset of symptoms to receive clopidogrel (300 mg immediately followed by 75 mg once daily, n=6,259 patients) or placebo (n=6,303 patients) in addition to aspirin for 3 to 12 months. It showed that DAPT versus aspirin alone significantly reduced the composite outcome of cardiovascular mortality, non-fatal MI or stroke (9.3% versus 11.4%; p<0.001). However, the rate of major bleeding was higher in the clopidogrel group (3.7% versus 2.7 %; p=0.001).42
Perhaps one of the most ironic features of modern PCI practice is that, although we have never checked the individual responses of our patients to their antiplatelet therapy in routine practice, it was concerns over frequent ‘hyporesponders’ to clopidogrel that provided the main stimulus to the development of more potent inhibitors of the P2Y12 pathway, particularly ticagrelor and prasugrel. As expected, these agents have been shown to be more effective than clopidogrel at reducing ischaemic events in ACS populations, but with an increased associated bleeding risk.43,44 In NSTEMI patients, the evidence favours prasugrel compared to ticagrelor, with lower incidence of death, MI or stroke and no significant difference in the incidence of major bleeding between the two groups, and this is reflected in the guidelines.27,45
The Role of Anticoagulants in Mitigating the Stroke Risk in AF
Description of Thromboembolic Risk in AF
AF is one of the most important modifiable risk factors associated with acute stroke.45,46 In the US, incidence of AF among all new hospital admissions with ischaemic stroke is 10–12%.47 Accordingly, risk stratification for the risk of stroke in AF and early initiation of therapy that aims to reduce the risk of AF-associated stroke is a crucial component in the management of this arrhythmia.
Various risk factors have been identified from epidemiological studies and clinical trials that affect the increasing risk of stroke in AF patients. The original CHADS2 score has been further refined, and currently the CHA2DS2−VASc score is recommended.48,49 The CHA2DS2−VASc score is well validated. For example, in a cohort study of 73,538 patients with non-valvular AF, the rate of thromboembolism per 100 person years in ‘low-risk’ patients (score=0) was 0.78 (0.58 to 1.04) at 1 year.50
There are also several tools that stratify bleeding risk and score the balance between thrombotic and bleeding risks, including the HAS-BLED score, HEMORR₂HAGES score and ATIRA score, of which HAS-BLED has been shown to outperform other risk scores.51
Clinical Evidence for Stroke Reduction with Warfarin and Non-vitamin K Oral Anticoagulants
Currently, chronic OAC treatment is the most effective available prophylactic approach in patients with AF at risk of thromboembolic events. Its efficacy in AF patients was first evaluated in 1996 in the SPAF-III trial.52 In a systematic review of sixteen trials that included a total of 9,874 patients, warfarin (six trials, 2,900 participants) reduced stroke by 62%; absolute risk reductions were 2.7% per year for primary prevention and 8.4% per year for secondary prevention.53 Unfortunately, but predictably, warfarin use was associated with a significant increase in the risk of major bleeding, including haemorrhagic stroke. The risk of major haemorrhage with warfarin when compared to aspirin was 2.2 versus 1.3 per 100 patient years (HR 1.71; 95% CI [1.21–2.41]).54
Given the challenges associated with warfarin monitoring, dose adjustment and interaction with diet and other drugs, the development of NOAC has been welcomed by patients and health workers. The DOACs, such as dabigatran, apixaban, edoxaban and rivaroxaban, have been shown to be equivalent to warfarin in the prevention of ischaemic stroke in non-valvular AF and might have a better adherence profile with less bleeding risk. In the RE-LY trial, 18,113 patients with AF were randomised to receive dabigatran (at doses of 110 mg or 150 mg twice daily) or warfarin (target international normalised ratio 2–3). There was a similar rate of stroke and systemic embolisation with lower rates of major haemorrhage in the dabigatran group. Rates of the primary efficacy outcome (stroke or systemic embolism) were 1.69%/year in the warfarin group, 1.53%/year in the group that received 110 mg of dabigatran (p<0.001) and 1.11%/year in the group given 150 mg of dabigatran (p<0.001). The rate of major bleeding was 3.36% per year in the warfarin group as compared with 2.71%/year in the group receiving 110 mg of dabigatran (p=0.003) and 3.11%/year in the group receiving 150 mg of dabigatran (p=0.31).55
In the ROCKET AF trial, rivaroxaban was non-inferior to warfarin for the primary efficacy endpoint of stroke and systemic embolism (2.1% versus 2.4% per year; p<0.001 for non-inferiority) and there was no difference between the two groups in terms of all bleeding events (14.9% versus 14.5%; p=0.44) and major bleeding events (3.6% versus 3.4%; p=0.58), with significant reductions in intracranial haemorrhage (0.5% versus 0.7%, p=0.02).56 Similarly, in the ARISTOTLE trial, apixaban was superior to warfarin in reducing risk of stroke and thromboembolism in AF patients (1.27% versus 1.6%; p=0.01) and had lower rates of major bleeding (2.13% versus 3.09%; p<0.001) including intracranial bleeding (0.33 versus 0.8%; p≤0.001).57,58 In ENGAGE-TIMI 48, edoxaban was associated with significantly lower rates of major bleeding and death from cardiovascular causes (3.17 versus 2.74%; p=0.01) compared with warfarin and was non-inferior with respect to stroke events and systemic embolism.55
In both the ESC 2020 and 2019 ACC/AHA/HRS guidelines there is a class I recommendation for choosing an NOAC over warfarin for stroke prevention in AF patients.3,7
Mechanism of Anticoagulant Action of Warfarin and Novel Oral Anticoagulants
Warfarin acts as an OAC by depleting the reduced form of vitamin K (acting as a cofactor for vitamin K-dependent coagulation factors) by blocking the function of the vitamin K epoxide reductase complex in the liver.59
Rivaroxaban and edoxaban both reversibly and highly selectively bind human factor Xa and the inhibition of prothrombinase complex-bound and clot-associated factor Xa results in a reduction of the thrombin burst during the propagation phase of the coagulation cascade.60 Similarly, apixaban reversibly and highly selectively inhibits free- and clot-bound factor Xa.61 Dabigatran is a direct factor IIa inhibitor and is a prodrug activated in the liver.61 Importantly, OAC and NOACs do not directly affect platelet aggregation induced by collagen, adenosine diphosphate or thrombin, but do have an effect by inhibiting factor Xa, which indirectly interferes with platelet activation and aggregation induced by thrombin.60,61
What is the Evidence for Antiplatelet Activity for Warfarin and Novel Oral Anticoagulants?
The inter-relationship between clotting and platelet-mediated thrombus formation is complex. Platelet activation and granule release occur due to a variety of agonists, as discussed above. The consequence of this activation can be a positive feedback loop in which released and generated molecules such as ADP and thromboxane A2 perpetuate and amplify the process of activation and aggregation.34,35 Concurrent with platelet activation and degranulation via a variety of pathways, the coagulation pathway cascade is activated via the generation of factor Xa and Va, leading to thrombin activation. Thrombin converts fibrinogen into fibrin. In addition, thrombin activates protease-1-activated receptor (PAR-1) and PAR-4 on platelets, which, in turn, enhances platelet aggregation.35,36 Platelet activation can be assessed using the surrogate of urinary excretion of 11-dehydro-thromboxane (TxB2) and soluble glycoprotein VI (sGPVI), a protein involved in platelet activation.62 In a cross-sectional study, apixaban and rivaroxaban were compared to warfarin regarding platelet activation. At 3 months, urinary excretion of TxB2 was 6.5% with warfarin (p=0.197), −29% with apixaban (p<0.001) and −31% with rivaroxaban (p<0.001). sGPVI was significantly lower in patients treated with NOACs at 3 months; a finding not observed in the warfarin group. This study provides evidence that NOACs significantly inhibit urinary TxB2 excretion compared to warfarin, suggesting the potential antiplatelet properties of NOACs.25 Furthermore, Pujadas-Mestres et al. reported the effect of apixaban on platelet deposition and fibrin formation onto a thrombogenic surface with blood circulating at arterial shear rates in a cell model of coagulation primed by platelets.63 A therapeutic dose of 160 ng/ml (equivalent of 10 mg/day) significantly reduced thrombus formation, fibrin association and platelet-aggregate formation. Apixaban significantly prolonged thermoelectrometry parameters but did not affect clot firmness. Notably, these effects on platelets and fibrin formation were not observed with the lower dose of apixaban 40 or 10 ng/ml (equivalent to 2.5 mg twice daily or 2.5 mg once daily, respectively).63
Role of Anticoagulants in CAD
The risk of recurrent ischaemic events is higher in patients with ischaemic heart disease (chronic coronary syndrome and ACS) in comparison to patients who have cardiovascular risk factors but without established atherothrombosis. For example, in the REACH registry, a total of 45,227 patients with a history of either CAD, cerebrovascular disease or peripheral arterial disease or with multiple risk factors for atherothrombosis were followed for 4 years. Among patients with atherothrombosis, those with a prior history of ischaemic events (MI or stroke) at baseline (n=21,890) had the highest rate of subsequent ischaemic events. Patients with stable coronary, cerebrovascular, or peripheral artery disease (n=15,264) had a lower risk (12.2%); while patients without established atherothrombosis but with risk factors only (n=8,073) had the lowest risk (9.1%; p<0.001 for all comparisons).64
The potential efficacy of apixaban (5 mg twice daily) in reducing ischaemic events for patients who have had ACS when added to aspirin and clopidogrel was studied in the APPRAISE-2 trial. In 7,392 patients after a median follow-up of 241 days, the primary outcome of cardiovascular death, MI, or ischaemic stroke was neutral (7.5% apixaban versus 7.9% placebo; p=0.51). Moreover, there was an increase in Thrombolysis in MI (TIMI) major bleeding when compared with placebo (1.3% versus 0.5%; p=0.001).65
Warfarin
Previously, the role of VKAs in secondary prevention of recurrent cardiovascular (CV) events in patients with established CAD has been studied in comparison to aspirin alone. For example, one systematic review that included 20,000 patients indicated that the use of moderate-intensity oral anticoagulation (INR 2 to 3) alone, did not significantly reduce the rate of CV death, MI and stroke versus controls. However, major bleeding occurred in 3.5% of patients receiving OA versus no patients in the control group, with an odds increase of 7.67 (p<0.0001).66 However, with use of high-intensity OA (INR >2.8) alone, cardiovascular death, MI, or stroke occurred in 20.3% versus 30.3% of patients who received no therapy (p<0.0001); by contrast, major bleeding occurred in 4.6% of OA patients versus 0.7% in controls (p<0.00001).66
One Danish registry investigated the effectiveness and safety of adding antiplatelet therapy (aspirin or clopidogrel) to a VKA in a total of 8,700 patients with AF and stable CAD (defined as 12 months from an acute coronary event), over half of whom had had a PCI. Over a mean follow-up of 3.3 years, the crude incidence rates for MI/coronary death, thromboembolism and serious bleeding were 7.2, 3.8 and 4.0 events per 100 person years, respectively. The risk of MI/coronary death was similar between VKA plus aspirin and VKA monotherapy. The risk of thromboembolism was comparable in all regimens that included VKA, whereas the risk of bleeding increased when aspirin (HR 1.50) or clopidogrel (HR 1.84) was added to VKA.67
NOACs in Patients with CAD/PCI:
Does the Evidence Support the Guidelines to Stop Antiplatelets?
The introduction of NOACs has stimulated comparative investigations of aspirin and VKA for the management of stable ischaemic heart disease. Aspirin has been shown to significantly lower the relative risk of MACE in this population. For example, in one meta-analysis of 16 secondary prevention trials comparing aspirin and placebo, the rate of ACS was 4.3% versus 5.3% per year, respectively (p<0.0001).68 Previous studies have demonstrated that the use of VKA with or without use of antiplatelets in patients with stable CAD is associated with a substantially elevated bleeding risk, occurring in approximately 1–4% of patients.66,69 This has led to the investigation of NOACs in consideration for stable ischaemic heart disease, either in combination with antiplatelet agents or alone.
In the PIONEER AF-PCI trial, 2,124 patients with non-valvular AF who had undergone PCI with stenting were randomised in a 1:1:1 ratio to low-dose rivaroxaban 15 mg once daily plus a P2Y12 inhibitor for 12 months (group 1), very-low-dose rivaroxaban (2.5 mg twice daily) plus DAPT for 1, 6 or 12 months (group 2), or standard therapy with a dose-adjusted VKA (once daily) plus DAPT for 1, 6, or 12 months (group 3). There was lower bleeding risk at 12 months in both rivaroxaban groups (group 1 and 2) in comparison to the standard therapy group with warfarin (16.8% in group 1, 18.0% in group 2 and 26.7% in group 3; p<0.001). Furthermore, there was no difference in the rates of death from cardiovascular causes, MI or stroke in the three groups.22
In the RE-DUAL PCI trial, dabigatran was tested in comparison to VKA in patients with AF who had undergone PCI with 2,725 patients randomly assigned to either triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin (for 1 to 3 months) (triple therapy group) or dual therapy with dabigatran (110 mg or 150 mg twice daily) plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and no aspirin (110 mg and 150 mg dual therapy groups). The mean follow-up was 14 months. The incidence of the primary endpoint (major or clinically relevant non-major bleeding event) was 15.4% in the 110 mg dual therapy group compared with 26.9% in the triple therapy group (p<0.001 for non-inferiority; p<0.001 for superiority). Higher rates were observed in the 150 mg dual therapy group: 20.2% versus 25.7% in the corresponding triple therapy group (p<0.001 for non-inferiority). The risk of thromboembolic events was however non-inferior in the DAPT groups when compared with the triple therapy group.70 Thpan study has therefore shown a lower bleeding risk for NOAC agents with non-inferiority in thromboembolism risk when used with either single or dual APT. However, in the context of this review, it is notable that it is not clear whether using it alone without any antiplatelet would yield the same effect on reducing thromboembolic events.
In the AUGUSTUS trial, the role of dual therapy (VKA or apixaban plus P2Y12 inhibitor) compared with triple therapy (VKA or apixaban plus aspirin and P2Y12 inhibitor) among patients with AF who had an ACS or had undergone PCI was evaluated. The primary outcome was major or clinically relevant non-major bleeding with 4,614 patients randomised in a 2 × 2 factorial design to either receive apixaban plus P2Y12 inhibitor or a VKA plus P2Y12 inhibitor and to receive aspirin plus dual therapy or matching placebo plus dual therapy for 6 months.
Clinically relevant major or non-major bleeding was noted in 10.5% of the patients receiving apixaban as compared with 14.7% of those receiving a VKA (p<0.001 for both non-inferiority and superiority) and in 16.1% of the patients receiving aspirin (triple therapy) as compared with 9.0% of those receiving placebo (dual therapy; p<0.001). Moreover, patients in the triple therapy group had similar incidences of death or hospitalisation and ischaemic events when compared to the dual therapy group.24 The risk of definite/probable/possible stent thrombosis was 1.6% within 6 months, with 80% occurring within 30 days. The number of patients with definite or probable stent thrombosis at 6 months was low in all groups. In the dual therapy arm, events were n=13 (0.74%) for apixaban and n=17 (0.97%) for VKA and in the triple therapy arm n=11 (0.63%) for aspirin and n=19 (1.08%) for placebo (p=NS). For apixaban plus aspirin, apixaban without aspirin, VKA plus aspirin and VKA without aspirin, the events were n=5 (0.57%), n=8 (0.91%), n=6 (0.69%) and n=11 (1.26%).24 As expected, the study had limited power to detect treatment effects on stent thrombosis rates. Again, it should be noted that in this trial, NOAC was not tested as the sole agent in the post-PCI patient subgroup.
The ENTRUST-AF PCI trial randomised 1,506 patients from 4 hours to 5 days after PCI to either edoxaban and aspirin or VKA and aspirin for 12 months. The rate of major bleeding or clinically relevant bleeding was 17% in the edoxaban group and 20% in the warfarin group (p=0.001 for non-inferiority; p=0.1154 for superiority) and the primary ischaemic efficacy endpoint was similar between groups.23 Again, there was no anticoagulant-only group.
Overall, these four randomised controlled trials have demonstrated that, in patients with AF and ACS or PCI, treatment with DOAC and P2Y12 inhibitor resulted in a lower bleeding risk profile than triple therapy (VKA, P2Y12 inhibitor and aspirin) with no difference in the ischaemic endpoints. Notably, however, they did not study the efficacy of OAC alone beyond 12 months post PCI.
The evidence base supporting the efficacy of OAC therapy alone without an antiplatelet agent, beyond 12 months after PCI in AF patients is relatively sparse. The first trial to address this question was the AFIRE trial and even this study did not do so in a specific population of post-PCI patients with AF.71
The AFIRE multicentre trial included 2,236 patients with AF who either underwent PCI (1,564 patients; 70.6%) or coronary artery bypass graft surgery (CABG) >1 year before (252 patients; 11.4%) or had known CAD without need for PCI. Thus, this was a markedly heterogeneous population. Patients were randomised to rivaroxaban alone or in combination with an antiplatelet agent (70% aspirin) and follow-up was 24 months.71 The primary efficacy outcome was a combination of all-cause mortality, MI, stroke, unstable angina requiring revascularisation or systemic embolism. The composite occurred in 4.1%/patient year of the rivaroxaban monotherapy group compared with 5.8%/patient year of the rivaroxaban/antiplatelet group (non-inferiority p<0.0001). The primary safety outcome – major bleeding – occurred in 1.6%/patient year of the rivaroxaban monotherapy group compared with 2.8%/patient year of the rivaroxaban/antiplatelet therapy group (p=0.01).17 Interestingly, the rate of MI in the rivaroxaban monotherapy group was 0.59%/patient year, while in the combination group it was 0.37%/patient year (p=0.778) and the rates of unstable angina requiring revascularisation was 0.59%/patient year in the monotherapy group versus 0.84%/patient year in the combination group (p=0.997). This trial was powered for non-inferiority and the headline outcome was that rivaroxaban monotherapy was non-inferior to combination therapy in terms of efficacy to prevent ischaemic/thromboembolic events, while being associated with significantly less major bleeding.72 Thus, from the perspective of this review, AFIRE provides some circumstantial evidence that the rivaroxaban without an antiplatelet agent is safe in reducing ischaemic events beyond a year after PCI. However, there are notable limitations in the trial and assuming that this can be translated into a routine policy in which antiplatelets are not required beyond a year in PCI patients on DOAC is highly questionable. First, the trial population included not just patients with PCI but also CABG patients and others who had not had PCI. Therefore, it was not powered to answer the question about the strategy in PCI patients specifically. Second, the time from PCI to recruitment into this trial was variable from any period beyond 1 year post PCI. Third, the tested strategy in AFIRE only relates to a 2-year period: given that most stent thrombosis occurs beyond 1 year, is continuous and does not reduce over time from PCI, this trial does not provide reassurance that post-stent patients are safe on a DOAC as monotherapy beyond the tested period.
OAC-ALONE was an open-label, multicentre trial that randomised 690 patients with AF beyond 1 year after stenting to OAC alone or combined OAC and single antiplatelet (85% aspirin). The primary endpoint included all-cause death, MI, stroke, or systemic embolism, and median follow-up was 2.5 years. The OAC employed was warfarin in 75.2% and a DOAC in 24.8% of patients.73 The composite primary endpoint was neutral and occurred in 54 patients (15.7%) in the OAC-alone group and in 47 patients (13.6%) in the combined OAC and APT group (p=0.20 for non-inferiority; p=0.45 for superiority). The secondary endpoint, a composite of the primary endpoint or major bleeding, occurred in 67 patients (19.5%) in the OAC-alone group and in 67 patients (19.4%) in the combined OAC and APT group (p=0.016 for non-inferiority; p=0.96 for superiority).73 Patients on OAC alone had a numerically higher rate of MI and stent thrombosis than OAC plus antiplatelet (2.3% versus 1.2% and 0.58% versus 0%, respectively), but none of these differences were statistically significant.
Thus, again this trial provides only minimal reassurance that a strategy of OAC without antiplatelet therapy in patients beyond 1 year after coronary stent insertion has a solid evidence base for routine clinical practice. Of note, this study was terminated prematurely due to low recruitment and was thus underpowered. Furthermore, the numerical differences in rates of MI and stent thrombosis events represent food for thought, especially since the same observation was seen in the AFIRE trial.
What Do the Current Guidelines Recommend?
The relative and combined value of OAC and APT in patients who have had PCI in the context of AF is complex and inevitably clinicians will look to clinical guidelines for guidance. The ESC guidelines for treatment of AF, MI and the use of DAPT is covered in a focused update on DAPT published in 2017, the 2018 guideline on MI and the 2020 ESC and European Association for Cardio-Thoracic Surgery (EACTS) guideline on the diagnosis and management of AF.8,27 The intersection of management between the two clinical conditions is covered in the US by the 2020 ACC Expert Consensus Decision pathway and by the 2019 focused update of the 2014 AF guideline from the ACC, AHA, and HRS.8,26
The ESC 2020 AF guideline recommends dual therapy, including OAC plus P2Y12 inhibitor, as a level Ia recommendation in patients with ACS or chronic coronary syndrome undergoing uncomplicated PCI. The recommendation on the duration of triple therapy is for up to 1 week post PCI then to discontinue aspirin and continue dual therapy (clopidogrel plus OAC) for up to 12 months in ACS patients, then DOAC monotherapy afterwards if the patient has not suffered recurrent ischaemic events in the interim with a recommended level of evidence of Ia.
In patients with chronic coronary syndrome undergoing uncomplicated PCI the recommended duration of dual therapy is up to 6 months followed by OAC alone with a level of evidence Ia.
In regard to oral anticoagulation choice, NOAC is preferred instead of warfarin when combined with an antiplatelet (level Ia).7 The ESC 2017 focused update on DAPT in CAD patients recommends dual therapy with OAC and one antiplatelet agent (aspirin or clopidogrel) beyond 1 year in patients with a very high risk of coronary events (Box 1).27
The recommendation on discontinuation of antiplatelets after 1 year from the 2017 ESC guidelines are derived from the 2014 Danish registry (as mentioned above) that reported that OACs alone are superior to aspirin post-ACS, and a combination of OAC with aspirin was not more effective at reducing ischaemic events but was associated with excess bleeding.27,67
The ACC 2020 expert consensus document recommends 12 months of anticoagulation with a single antiplatelet, preferably using clopidogrel in post-PCI patients following ACS and 6 months post-PCI for chronic coronary syndrome for patients who require lifelong anticoagulation.8,26 Thereafter, it is recommended that OAC therapy alone could be used long term, except in patients at high risk for thrombotic events but with a low risk of bleeding. In this cohort, they recommend continuation of OAC plus a single antiplatelet beyond 12 months.
This consensus recommendation is derived from recent data from the AFIRE and OAC-Alone trials.26,72,73
Conclusion
The risk of ST in patients who receive a DES is attractional, and the VLST event is the commonest (i.e. beyond 12 months after the stent insertion). Stent thrombosis and acute MI are most commonly mediated by platelet activation and aggregation. The evidence that DOACs have direct antiplatelet activity is relatively sparse. The clinical trial evidence that OAC in general and DOAC in particular, can safely be used without antiplatelet agents in stent patients beyond 1 year is weak and this strategy has never been specifically tested in this population. Therefore, should single antiplatelet therapy with DOAC be considered for the majority of patients 1 year after stent insertion (Figure 1)?