Approximately 1% of the UK population have a diagnosis of a severe mental illness (SMI). A diagnosis of SMI is associated with a decrease in life expectancy of up to 20 years compared with the general population.1 Although part of this mortality gap is due to complications of psychiatric disease, such as suicide, the major cause of death is cardiovascular (CV) disease (CVD).2,3
The increased risk of CVD in patients with SMI is seen in both sexes and across adult age groups and ethnicities (although with variations within groups).2,4 Importantly, a diagnosis of SMI predisposes to early CVD, with patients with bipolar disorder (BD) developing CVD over a decade earlier than non-psychiatric controls.5 Patients with SMI have not benefitted from the same reductions in CV risk factors, CVD and CV death otherwise seen in Western populations over the past decades, and, as such, the mortality gap persists.6,7
The term, SMI, can be used to encompass a range of mental illnesses. In this review, we use the term, SMI, to denote schizophrenia and BD. This narrative review will focus on the relationship between SMI and CVD, particularly coronary artery disease (CAD), along with CVD management strategies in this group, including coronary revascularisation.
Evidence for Accelerated Atherosclerosis in Severe Mental Illness
The American Heart Association recognises that SMI predisposes to early CVD via accelerated atherosclerosis.8 Patients with schizophrenia present with acute MI approximately 10 years earlier than those without psychiatric disease, while patients with BD develop incident CVD up to a decade earlier than non-psychiatric controls and have a standardised mortality rate (SMR) of up to 8, with the highest relative risk from CAD seen in patients aged <20 years.9,10 In addition to the cardiometabolic abnormalities already discussed, it has been suggested that other contributing factors to CV risk include endothelial dysfunction and oxidative stress.
Endothelial dysfunction is strongly and independently associated with atherosclerosis and CVD.11 Patients with schizophrenia have impaired endothelial and microvascular peripheral blood flow response, while patients with BD have elevated levels of biomarkers associated with endothelial function.12–17 There is also strong evidence for increased oxidative stress in SMI. A meta-analysis of 44 studies found increased markers of oxidative stress in patients with BD, seen in unmedicated patients, but not in those receiving effective antipsychotic therapy.18 Patients with schizophrenia also demonstrate increased levels of markers of oxidative stress, this is not modulated by the effect of antipsychotic treatment.19,20
Risk Factors for Cardiovascular Disease
In contrast to the wider populations, temporal trends of risk factors for CAD have not improved in people with SMI. One example of this is cigarette smoking; rates of smoking remain higher in those with schizophrenia compared with the general population.21 This is despite evidence that multimodality interventions to aid smoking cessation are effective in patients with SMI, including clinician advice, cessation counselling and nicotine replacement.22 Specific medications to aid smoking cessation; for example, bupropion and varenicline, are safe to use and effective in patients with SMI.23,24 The WHO specifically cites misconceptions from mental health professionals about patients’ willingness and ability to quit smoking as a key challenge for decreasing smoking among patients with SMI.25
One epidemiological study in the US demonstrated a significantly greater prevalence of hypertension among individuals with BD compared with controls (OR 4.95; 95% CI [4.27–5.75]). In addition, on average, people with BD who developed hypertension were >10 years younger than non-psychiatric controls.26
There is strong evidence for an association between metabolic syndrome and SMI, with evidence of glucose dysregulation and raised triglyceride levels at first presentation.27,28 In patients with schizophrenia, there is an interaction between the number of psychotic episodes and markers of metabolic syndrome, such as abdominal obesity, cholesterol and triglyceride levels; patients with multiple psychotic episodes are at higher risk compared with first-episode and drug-naïve patients.29 This is also seen in people with BD and is at least partly attributable to psychiatric medications.30
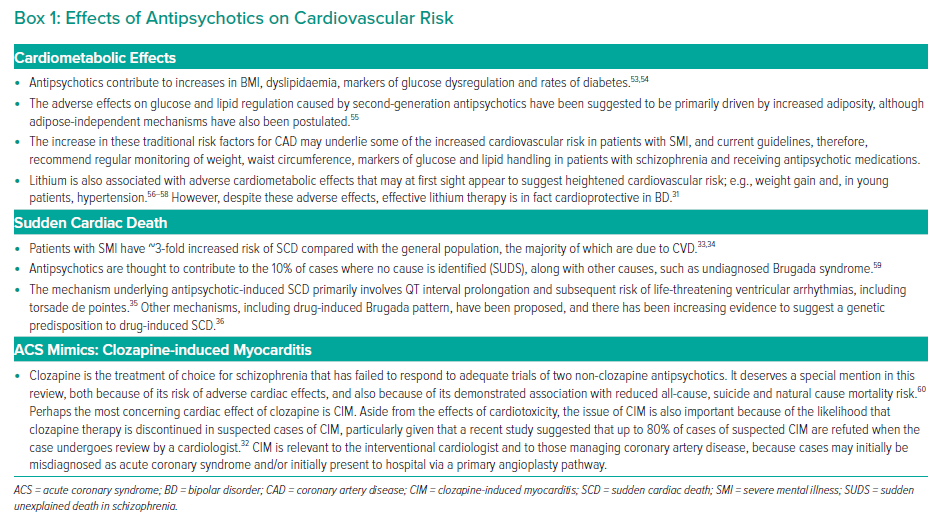
Psychiatric medications are recognised mediators of CV risk. This is largely due to medication side-effects, such as weight gain and glucose dysregulation, but also because psychotropics may interact with commonly used cardiovascular pharmacotherapy. For example, lithium toxicity may develop when used with angiotensin-converting enzyme inhibitors, while there is increased risk of hypokalaemia and torsades de pointes when used with diuretics.31 Clozapine, the gold-standard antipsychotic drug for patients with treatment-resistant schizophrenia, has significant adverse metabolic effects and is also relevant to acute cardiac care due to the side-effect of clozapine-induced myocarditis, which may present as an acute coronary syndrome (ACS) mimic. It should be noted, however, that clozapine-induced myocarditis is both rare and often overdiagnosed.32
SMI is associated with an increased risk of sudden cardiac death.33,34 While the majority of this phenomenon is due to underlying CVD, approximately 10% of cases are attributed to antipsychotics due to mechanisms, such as QT prolongation and drug-induced Brugada syndrome.35,36
However, despite the particular risk profiles noted above, lithium and antipsychotics have overall benefits on all-cause mortality and cardiovascular mortality.31,37 Box 1 shows a list of CV effects of antipsychotic medications.
Diagnosis of Coronary Artery Disease in Patients with Severe Mental Illness
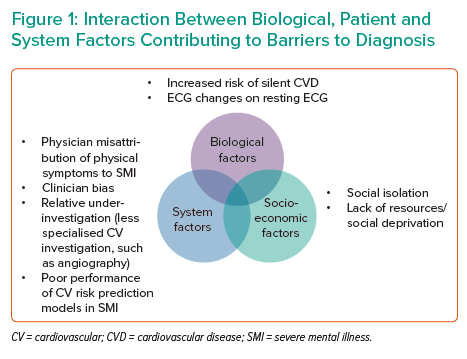
Several features of SMI can make accurate diagnosis of CAD difficult. These can be categorised into biological factors, socioeconomic factors and system factors (Figure 1).
- Biological factors. Patients with SMI and ACS are less likely than non-psychiatric controls to present with chest pain, and there is an increased risk of ‘silent’ CAD.9,38,39 Diagnostic uncertainty in patients with ACS may also be affected by ECG abnormalities in patients with SMI taking antipsychotics (patients with schizophrenia are more likely to have benign ST elevation on a resting ECG in the absence of an acute ST-elevation MI; STEMI).40 While Danish registry data of patients with SMI found that patients with SMI were less likely than non-SMI controls to have an abnormal ECG, there was a significant interaction between SMI and ECG abnormalities on CV death.41 Among patients with SMI, abnormalities indicating prior pathology are more commonly encountered, may make ECG interpretation more challenging and, as would be expected, result in poorer outcomes.
- Socioeconomic factors, such as social isolation and deprivation, may be relevant.42 Indeed, social isolation highlights the importance of involving relatives and carers of patients with SMI wherever appropriate, and is considered to be a key facilitator in providing effective care by general practitioners.42
- System factors include misattribution of physical symptoms to the primary psychiatric disease.43 There may be difficulty obtaining high-quality cardiac investigations on patients admitted to psychiatric institutions and/or relative inexperience or underconfidence of psychiatrists in interpreting clinical data from such investigations. Other system factors include barriers to accessing specialist secondary care, both for initial assessment and investigations, but also specialist follow-up.44 It should also be recognised that widely-used models for CVD risk prediction perform poorly in patients with SMI.45 Clinician bias should also be highlighted; medical physicians are more likely than psychiatrists to have a negative attitude towards the patient with SMI.46
Some difficulties with CAD diagnosis in patients with SMI may be attributable to a combination of the factors discussed above. For example, in the acute setting, patients with SMI diagnosed with an acute coronary syndrome are more likely to have pathological Q waves on presentation, suggestive of a full thickness MI, which may be a consequence of reduced chest pain signifiers, delayed presentation and/or delayed investigation.9
The diagnostic challenges aforementioned should highlight the importance of early involvement from cardiology teams, and additional use of other investigations in the acute work-up of patients with SMI presenting with possible ACS (e.g. use of bedside echo to identify regional wall motion defects that may correspond with ECG abnormalities). Clinicians working in a centre without on call cardiology should have a low threshold for discussing these patients with the local tertiary centre.
Management of Coronary Artery Disease in Patients with Severe Mental Illness
Retrospective data from Denmark suggest that approximately 4% of patients with CAD also had a diagnosis of SMI.47 Consistent with the above risk factors, patients with SMI were younger, with higher rates of smoking and diabetes. They also had a longer duration of symptoms. Despite this, in-hospital management of the incident ACS did not differ between the two groups, and in-hospital mortality was similar. However, patients with SMI were less likely to receive evidence-based therapies during follow-up and, compared with the control group, further major adverse cardiac events were more likely during the follow-up period (despite adjustment for the increased baseline risk in the SMI group).
A large retrospective sample (~3,000,000 with 1% SMI prevalence) assessed trends in the management and outcome of patients with SMI compared with controls in the US from 2003 to 2013.48 As in the Danish study, patients with SMI and ACS were younger and more likely to be women, with higher rates of diabetes and obesity compared with those without SMI. However, during the period studied, patients with SMI failed to derive the benefits that the non-SMI control group did in terms of decreased incidence of STEMI and decreased in-hospital mortality. Although both SMI and non-SMI groups had increasing revascularisation rates over the decade studied, this trend was more pronounced in the non-SMI group. Overall, patients with SMI were less likely to undergo coronary revascularisation, but a diagnosis of SMI was not associated with increased in-hospital mortality.
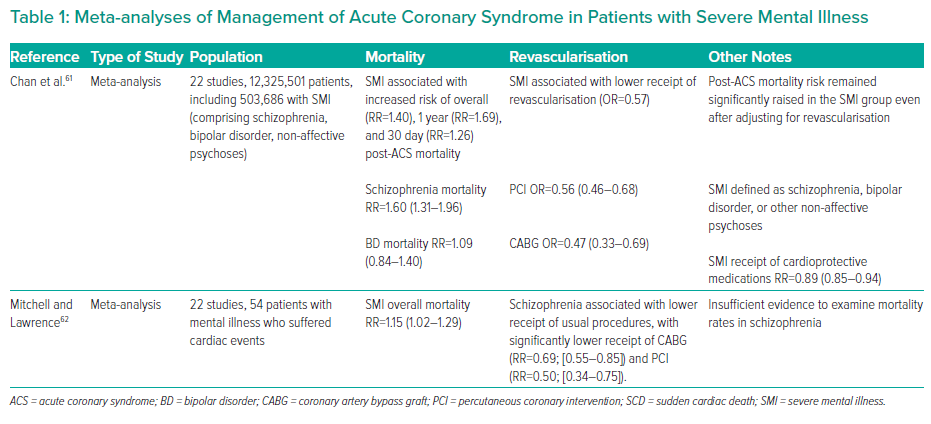
In a Swedish cohort (~300,000), patients with schizophrenia were more likely to present with an atypical symptom profile (i.e. without reported chest pain or breathlessness) and were less likely to undergo invasive investigation.9 Those who did undergo invasive investigation with coronary angiogram were less likely to be managed with percutaneous coronary intervention or coronary artery bypass graft compared with the control group, despite having similar rates of coronary anatomy. Of note, patients with schizophrenia had numerically higher rates of inconclusive angiographic findings (50.5% versus 46.5% in the control group). Over a 5 year follow-up period, patients with schizophrenia had an approximately twofold increase in major adverse cardiac events and all-cause mortality compared with those without schizophrenia. Table 1 shows a summary of meta-analyses relevant to revascularisation in patients with SMI and ACS.
The finding that patients with SMI are less likely to undergo invasive investigations and management raises the question of why. Is it that patients with SMI are more likely to be considered inappropriate for an invasive strategy and are therefore not offered the relevant investigations? Or are patients with SMI offered the tests, but are more likely to refuse them? A small Danish case–control study attempted to address these questions by completing in-depth reviews of patients with and without schizophrenia who had an ACS event. They found that patients with schizophrenia were significantly less likely to be offered and accept examinations and treatments for incident ACS. However, the study was underpowered to detect whether this primary endpoint was due to a lack of offer on behalf of the clinician or acceptance on behalf of the patient.49
Secondary Prevention
Given that patients with SMI have both increased rates of CV risk factors and increased incidence of CV disease, it stands to reason that these patients should benefit more from the standard secondary prevention medications that are offered to the general population following a diagnosis of CV disease. However, registry data suggest that patients with SMI discharged from hospital after an ACS event are less likely to receive P2Y12 inhibitors, statins or drugs acting on the renin–angiotensin axis.9
Strategies for Management and Revascularisation of Coronary Artery Disease in Patients with Severe Mental Illness
Assuming Appropriateness for Invasive Approach Unless Proven Otherwise
Many of the deficiencies in the management of CAD in patients with SMI occur for obvious and at times insurmountable reasons. Take for example the patient with severe active psychosis who may not be able to communicate their cardiac symptoms because of thought disorder or who may not be able to tolerate invasive investigations and/or management because of paranoid beliefs or agitation. Indeed, this may be the scenario that many cardiologists or cardiothoracic surgeons will first think of when considering patients with SMI. However, it must be emphasised that symptoms of SMI exist on a spectrum of severity and, therefore, most patients with SMI will be, or may become, suitable for an invasive strategy. The starting point in managing patients with SMI who present with CAD must, therefore, always be that they should be considered for the full range of cardiac interventions, with restrictions on therapeutic approaches (with associated potential adverse impact on prognosis) taking place only after careful consideration of best interests, and inclusion of liaison psychiatry to help manage psychiatric symptoms.
Removing Structural Impediments to Effective Cardiovascular Care
Due to the highly protocolised nature of acute cardiovascular care (e.g. primary angioplasty pathways, acute heart failure care) there should in theory be fewer impediments to patients with SMI receiving prompt, evidence-based care. However, this assumption is dependent on patients with SMI entering the pathway in the first place. In the Swedish ACS cohort discussed above, patients with schizophrenia presenting with ACS were not noted to suffer from any ‘system delay’ compared with patients without schizophrenia.9 However, ‘system delay’ was defined as time from ECG to percutaneous coronary intervention, a metric that relies on a patient undergoing ECG investigation in the first place, something that could in theory be less likely in patients with schizophrenia, given that they are less likely to complain of chest pain as the presenting symptom. On a more general note, a clinical audit of CV risk factor management and care provision to patients with SMI can be a powerful tool to identify inadequacies in management for patients with SMI.
Interventional Strategies
No data exist to support any deviation from a standard interventional approach in revascularisation of CAD in patients with SMI, but consideration is needed as to how to improve access to such pathways. It is intuitive that, as in any patient with significant comorbidity, multidisciplinary discussion is of great importance. It stands to reason that the management of CAD in patients with SMI should be discussed in a ‘Heart Team’ forum (with input from his/her psychiatrist where appropriate), particularly if the management offered deviates from that which would be offered to a patient without psychiatric disease.
Potential reasons to deviate from a standard revascularisation strategy may include:
- Concerns regarding medication compliance. This may lead to shorter durations of dual-antiplatelet therapy and/or use of absorbable polymer or polymer-free drug-eluting stents, or drug-eluting balloon therapy where possible. Most modern drug-eluting stent platforms can accommodate an abbreviated period of dual-antiplatelet therapy as short as 1 month; however, this does not remove the need for lifelong single antiplatelet therapy following drug-eluting stent implantation to avoid stent thrombosis.50,51
- Concerns regarding current psychiatric status and risk of deterioration in symptoms; for example, after cardiac surgery. In such circumstances, advice from psychiatry as to how to minimise these risks is invaluable, given the evidence that the use of antipsychotics is associated with increased adherence to cardiometabolic medications in people with schizophrenia.52
Gaps in the Current Literature: Future Research Required
As per the American Heart Association position statement categorising BD, along with major depressive disorder, as conditions predisposing to accelerated atherosclerosis and early CVD, there are clear unanswered research questions to be addressed; for example what is the effect of sex differences and can non-pharmacological interventions targeted at both mood and CV risk (e.g. exercise) have a dual beneficial effect?8 Furthermore, does enhanced prescribing and monitoring of primary and/or secondary prevention medications improve outcomes in patients with any SMI? As in all areas of CV medicine, randomised controlled trial evidence, supplemented by large-scale observational data, will be vital in answering these questions.
ECG Abnormalities
Given the association between ECG abnormalities and CV death in patients with SMI, it has been suggested that SMI patients with ECG abnormalities but no pre-existing CV diagnosis may benefit from targeted CV investigation and management, thereby potentially preventing CV events before they occur. This is an interesting hypothesis, building on existing work that adding ECG data to conventional risk models can make CV prognostication more accurate and warrants further study.41
Data in the Primary Angioplasty Era
Little of the current body of literature describes ACS care in the era of primary angioplasty. Urban centres in the UK, with well-established and highly protocolised primary angioplasty pathways, and robust standardised data collection across all centres nationally (via British Cardiovascular Intervention Society/National Institute for Cardiovascular Outcomes Research), are the perfect setting to answer research questions surrounding performance of these networks in patients with SMI presenting with ACS. It is vitally important that structural barriers to effective healthcare are identified and then addressed for SMI patients with and/or at risk of CVD.
Conclusion
Patients with SMI are at increased risk of and from CAD, yet are less likely to receive evidence-based management. A proactive and multidisciplinary approach from the Heart Team is required to minimise this concerning treatment gap.