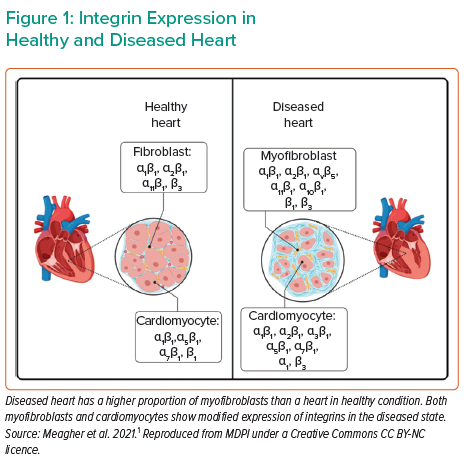
In cardiac disease resulting in severe heart failure, there are numerous molecular effectors translating the change in wall stress to cardiac remodelling. Various molecules act as sensors, reacting to mechanical stress and altering the mechanical properties of the heart by means such as cardiac fibrosis, and ultimately affecting cardiac contractility. Such sensors include integrins and their downstream products, with differential expression between healthy and diseased hearts (Figure 1). Cardiomyocytes express several integrin transmembrane receptors, including α1β1-, α5β1-, α7β1- and β1-integrin. These receptors are critical components of the pathway affecting Titin regulation, controlling the force and stiffness of myocytes. In cardiac fibroblasts, α1β1-, α2β1-, α11β1- and β3-integrin are involved in the response to mechanical stress, stimulating the production of collagen and extracellular matrix remodelling. In diseased myocardium, as in dilated cardiomyopathy (DCM), there is a change in integrin expression patterns in myocytes and fibroblasts.1
Dr Tschöpe hypothesised that effective prolonged unloading with Impella (termed ‘PROPELLA’) targets integrin-related cardiac remodelling pathways in patients with severe heart failure in non-classical cardiogenic shock (Society for Cardiovascular Angiography and Interventions [SCAI] A/B) and set out to test whether PROPELLA can be a bridge-to-recovery strategy for these patients, potentially avoiding the use of permanent mechanical circulatory supports such as durable left ventricular assist devices.
Using mass spectrometry, Dr Tschöpe’s team analysed cardiac biopsies from severe myocarditis patients with DCM bridged with PROPELLA. Samples were collected at baseline, during Impella support and 5–6 months after device explant. Principal component analysis demonstrated a marked shift in protein expression patterns across these time points. Quantitative polymerase chain reaction analysis of gene expression revealed that the expression of the integrin receptors, specifically α1-, α5-, α6- and α10-integrin, decreased significantly during unloading with Impella and returned to baseline levels after explanting the device. Similar to integrin receptor expression, immunohistochemical staining for four different inflammatory cell populations showed that immune cell presence decreased during active unloading time points. Interestingly, during unloading, collagen and matrix protein (e.g. vimentin) expression was reduced and remained reduced after Impella weaning. Phosphorylation of Titin, a critical component of myocardial stiffness, was increased during Impella therapy, along with protein kinase A and G activity, both of which are important for Titin phosphorylation; these changes were maintained after removal of the device. Together, the data indicate that unloading modifies integrin-related systems, including improving Titin phosphorylation, energy metabolism, collagen expression, inflammation and the immune system response.2
Dr Tschöpe noted that a mismatch can occur between mechanical and molecular unloading in a subset of patients, in whom mechanical improvements in conductance measurements are observed but integrin and immune signalling fail to respond to Impella unloading. Consequently, these patients exhibited no benefit after Impella weaning.
Dr Tschöpe concluded that PROPELLA may favourably alter cardiac remodelling in patients at risk of severe heart failure. Identifying non-invasive markers will help determine which patients may benefit from a bridge-to-recovery strategy with PROPELLA and which patients will not recover with prolonged unloading.