Coronary angiography and percutaneous interventions are now routinely performed via three access sites: the femoral, brachial and radial arteries. The first case of access to the arterial system was performed and described by Seldinger more than 50 years ago.1 Sones et al., using direct exposure of the brachial artery, performed selective coronary visualisation with subsequent suture closure of the arteriotomy.2 The widespread application of these procedures was greatly facilitated by the adoption of the Seldinger technique in conjunction with use of a sheath with a haemostatic valve. These technological innovations, coupled with the development of catheters designed to allow simple and efficient cannulation of the coronary arteries from the femoral artery, propelled cardiac catheterisation into a mainstream cardiac procedure.3 There are now over one million cardiac catheterisations performed in the US each year and the femoral artery remains the access of choice for most of the country. Advances in technology, technique and patient selection have improved non-access-related complications of coronary procedures, but femoral access is still associated with a bleeding or vascular complications rate of up to 10 % in some series of percutaneous interventions.4 Although manual compression remains the gold standard for achieving haemostasis, vascular closure devices (VCDs) have proved a safe alternative since their introduction in the early 1990s, now boasting a success rate of over 90 %. Multiple VCDs now address the major limitations of manual compression, including patient discomfort, increased time to haemostasis and ambulation, and increased requirements on catheterisation laboratory staff. The major facets of today’s technique of obtaining femoral arterial access and the mainstream devices used for vascular closure will be discussed in this article.
Femoral Artery Access
Prior to consideration of the femoral arterial approach, a detailed history and physical is a prerequisite, especially regarding conditions that may prevent a safe transfemoral procedure. This evaluation should focus on symptoms of peripheral vascular disease, prior lower extremity vascular surgery or previous transfemoral access. Additionally, the physical examination is an important step to identify femoral artery pulses and landmarks such as the inguinal crease, the superior anterior iliac spine and the symphysis pubis. A thorough inspection of the skin area during this time will allow identification of active groin infection which should preclude access at that site. Palpation and auscultation of the femoral artery is important, especially concerning sites of previous access or complication, and will allow assessment of potential scarring, aneurysm/pseudoaneurysm or evidence of previous surgery or injury. At this point, the patient is ideally supine and should demonstrate tolerance to this position during the examination, or alternative access should be considered.
A percutaneous procedure begins with choosing the arterial vascular access site most appropriate to ensure the success and safety of the principal diagnostic or interventional procedure. The location and size of the vascular access differ greatly between coronary and peripheral vascular procedures such as renal, iliofemoral and infra-inguinal interventions. The quality and success of appropriate femoral access have also been increasingly recognised, now that we have entered an era of large-bore equipment used in percutaneous support devices, aortic endografts and, more recently, transcatheter aortic valve implants. If transfemoral access is selected for the individual and the procedure, then attention to achieving optimal femoral artery access is important to allow use of appropriate-sized sheaths and minimise potential complications. Although serious complications of cardiac catheterisation remain quite low, location of the femoral artery puncture site outside the common femoral artery (CFA) has been shown to be among the most common causes of complication during the procedure.
Anatomical Considerations
The CFA arises from the external iliac artery, immediately behind the inguinal ligament. The exit of the CFA out of the retroperitoneum is identified angiographically by the inferiormost portion of the inferior epigastric artery, which wraps around the inguinal ligament and then runs upwards. The femoral artery proceeds through the femoral sheath over the underlying femoral head. The CFA then branches into the superficial femoral artery and profunda femoris. Arterial puncture sites above the inferior epigastric artery (‘high stick’) can result in life-threatening retroperitoneal bleeding, due to the location of the arteriotomy above the inguinal ligament, making manual compression and haemostasis more difficult.5 This is due to increased tissue between the arteriotomy and the skin and, more importantly, having to apply pressure to this site without the benefit of the femoral head to press upon. Puncture sites below the bifurcation (‘low stick’) result in cannulation of a smaller calibre vessel and increase the potential for pseudoaneurysm formation, as well as arteriovenous fistulae, due to the close proximity of femoral venous branches at this level in the thigh.
Optimal Common Femoral Access
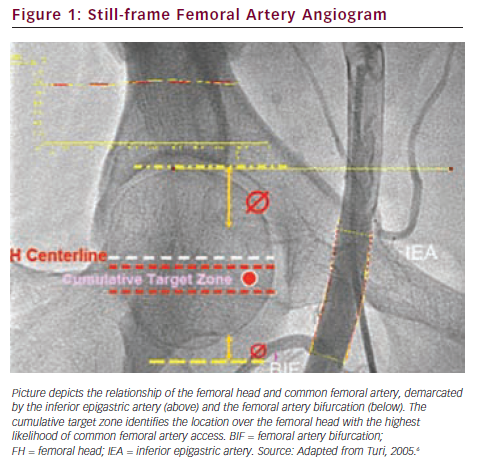
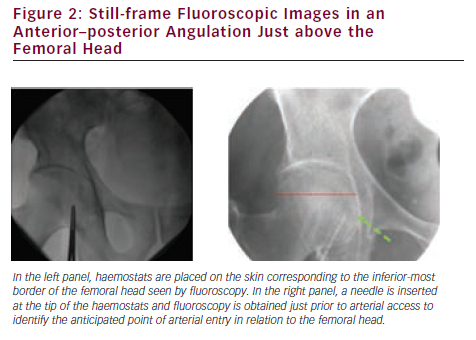
Use of bone landmarks is integral to obtaining common femoral access. Although palpation of the femoral head itself was initially practised, the location of the femoral head in reference to the access site on the skin is now most easily obtained using fluoroscopy. The anterior–posterior view of the femoral head under fluoroscopy has been divided into zones according to the anatomical probability of containing the inferior epigastric artery (upper quadrant) and the femoral artery bifurcation (lower quadrant). The ‘target zone’ (see Figure 1) is associated with the highest probability of obtaining a common femoral stick.6 The target zone, in the upper half of the lower portion of the femoral head, is located just below the midline of the femoral head and can reasonably be accessed using a ‘double fluoro’ technique (see Figure 2). This entails using a fluoroscopy image of forceps over the femoral head to identify the best skin entry site. As the needle is advanced towards the arterial pulse, but before entering the lumen, a second fluoroscopy image is obtained to ensure the needle will enter the target zone. Once used as the access point of choice, the skin crease is now felt to be unreliable for safely identifying the CFA as it does not often correlate with the inguinal ligament.
Use of Fluoroscopy
Fluoroscopic guidance has been widely adopted in clinical practice based on observations regarding the association of the majority of femoral artery bifurcations and the inferior border of the femoral head.7 Three studies have compared femoral artery access obtained using fluoroscopic guidance and by traditional use of anatomical landmarks.7–9 The incidence of obtaining CFA access was similar for both techniques, although there were statistically significant increases in low sticks with the traditional technique. These observations support use of either technique to guide femoral artery access, although it is not clear what effect training in the use of fluoroscopy to aid access prior to the study may have had on the study outcomes.
Ultrasound Guidance
Recently, operators have extended the technique of ultrasound-guided venous access to femoral arterial access. In this technique, the bifurcation is identified by an ultrasound probe and arterial access is obtained above the bifurcation. In a multi-centre prospective randomised single-blinded trial, ultrasound guidance compared with access obtained by traditional landmarks demonstrated a significantly lower rate of time required for access, as well as a lower rate of vascular access complications and number of low sticks, without a significantly increased number of high sticks.10 This technique appears to provide a reasonable alternative to fluoroscopy-guided femoral access with a minimal amount of training and additional equipment, although widespread adoption of this technique has been limited.
Sheath Insertion
Femoral artery access is commonly obtained with an 18-gauge needle advanced just a few millimetres into the vessel using an anterior single-wall stick. A 0.035 mm J-tipped wire can be fed into the needle into the CFA. The J-tipped wire should easily advance through the common iliac arteries and into the distal abdominal aorta. If there is resistance, then fluoroscopy may allow manipulation of the wire through a tortuous or diseased vessel. Occasionally, a curved transit catheter may provide additional support and manoeuvrability to successfully navigate the anatomy. In these cases, a long exchange wire should be employed for catheter exchanges for the remainder of the case to minimise arterial trauma. After wire position is achieved in the thoracic aorta, a small skin nick may be made to facilitate sheath placement without using excessive force or jeopardising the integrity of the sheath tip’s tapered edge, although this is not necessary with many 4 and 5 French (Fr) sheaths today. Scar tissue is seldom encountered, although it is common in patients with multiple previous transfemoral catheterisations. Although smaller (4–6 Fr) tapered sheaths usually traverse scar tissue without pause, occasionally a stiff-bodied wire can be exchanged through the sheath’s dilator to provide additional support for sheath insertion.
Femoral Angiography
Once access is obtained, immediate femoral angiography is useful for several reasons. The femoral angiogram provides visualisation of the arteriotomy location and identifies both high and low sticks. Additionally, bleeding and local complications can be identified and treated, or the procedure modified as needed. Closure device use is probably optimised when CFA access is obtained, and preparations for closure device use can be made before the procedure is completed. Finally, for those just beginning, a femoral angiogram provides immediate feedback of the access process and access location. The optimal femoral angiogram is obtained in 30–45° ipsilateral anterior oblique view with medial retraction of the proximal aspect of the sheath to adequately visualise the bifurcation and point of entry. This can be carried out with minimal contrast. In patients with reduced glomerular filtration rates the contrast load may be diluted with saline.
High Sticks
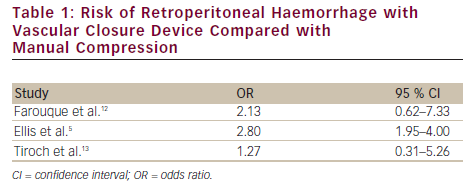
Knowledge of the arteriotomy site offers the potential advantage of estimating the approximate risk of peri-procedural bleeding and potentially saving the patient exposure to antithrombotic and anti-platelet therapy by not proceeding to percutaneous coronary intervention (PCI) (e.g. if a high stick is present). Several recent studies have assessed the risk of retroperitoneal haemorrhage with a high stick.5,11,12 In these three studies, there was an increased odds ratio of four- to eighteenfold for a high stick,5,11,12 although the absolute risk of a retroperitoneal bleed in the setting of a high stick may be as low as 2 %.13 Additionally, use of a VCD to close a high stick has been associated with an increased risk of retroperitoneal haemorrhage, from 1.3 to 2.8 times that of manual compression (see Table 1).5,12,13 These observations have prompted many clinicians to defer PCI and anticoagulation in cases of a high stick, unless clinical circumstances dictate otherwise.
Vascular Closure Devices
Access site-related bleeding remains the Achilles heel of procedures performed from a femoral access site. Fortunately, rates of access site bleeding have continued to decline over the past 10 years, due to multiple advances in pharmacology and equipment during this time.14,15 Nonetheless, peri-procedural bleeding has been associated with increased post-procedure mortality, although a precise mechanism for this association is still being sought. Despite advances in vascular access, the use of smaller sheaths and the availability of closure devices, manual compression continues to be the most commonly used technique for achieving haemostasis after coronary angiography and PCI. VCDs were clinically introduced in the 1990s to address the limitations of manual compression with the use of large-bore sheaths and intensive anticoagulation regimens, and have seen rapid evolution since that time. Although the early devices had failure rates above 10 %, several modifications of almost all devices have simplified use and substantially reduced failures.15
Indications for Use of Vascular Closure Devices
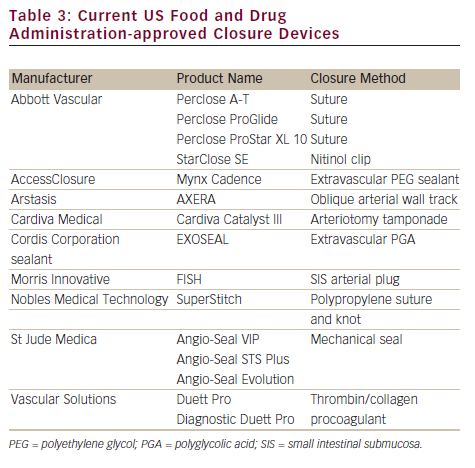
Manual compression can be safe and effective for achieving haemostasis, but is often associated with patient dissatisfaction issues such as delayed ambulation, local discomfort, increased time/personnel requirements and bleeding complications associated with suboptimal pressure upon the arteriotomy – especially in the context of large-bore sheaths and anticoagulation. There is no absolute contraindication to using a VCD, although device manufacturers provide strict instructions and guidelines for their optimal use (see Table 2). Generally, a CFA stick is strongly recommended as the preferred access site for closure. VCDs may be associated with ischaemic complications if deployed in the smaller-calibre branch vessels below the CFA. Use in small femoral arteries (<4–5 mm lumen) and arteries with moderate to severe atherosclerotic disease of the femoral artery has been shown to increase ischaemic complications as well. Diabetic and immunosuppressed patients have increased susceptibility to local infection and some centres advocate peri-procedural antibiotics, although there is no consensus on this practice. Additionally, analysis of data from over 1.5 million patients participating in the National Cardiovascular Data Registry (NCDR) demonstrated lower bleeding rates among VCDs when used in conjunction with bivalirudin, especially regarding those at high risk of bleeding complications.16
Classification of Vascular Closure Devices
VCDs can be classified according to the manner in which they achieve haemostasis. The most widely used devices use active approximation, in which the breach in the arterial wall at the arteriotomy site is closed with an intra-arterial anchor and extravascular collagen sponge creating an ‘arteriotomy sandwich’, a suture or a clip. Currently US Food and Drug Administration (FDA)-approved devices are listed in Table 3.17 These active approximation devices were used for haemostasis in 40 % of the 1.5 million PCIs between 2004 and 2008 in a large registry.16 Passive closure devices use extravascular sealant or other temporary mechanical means to facilitate manual compression. Although these devices may accelerate haemostasis, they are less commonly used than active approximation devices due to the comparatively longer time to achieve final haemostasis and ambulation. The three most commonly used and studied active approximation devices will be discussed below in greater detail.
Specific Vascular Closure Devices
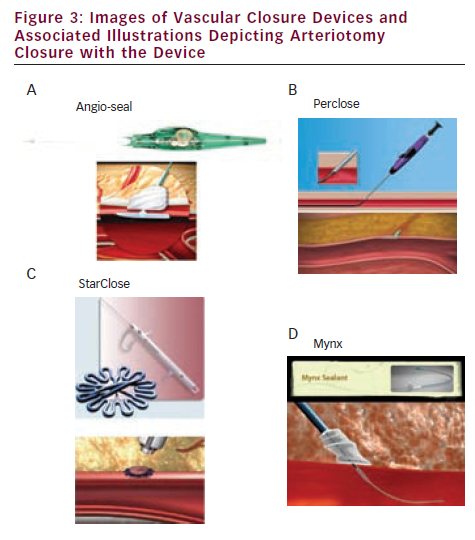
The Angio-Seal™ device (St Jude Medical, St Paul, MN) is the most commonly used closure device worldwide, with more than 15 million Angio-Seal devices deployed globally since its inception.18 This device has gained popularity through a high success rate and a relatively short learning curve for use. The device consists of an intraluminal anchor and an extravascular collagen sponge attached to the anchor via a bioresorbable suture. The components are all bioresorbable and resorption is chemically complete at three months. Haemostasis is achieved by active approximation and creation of an arteriotomy sandwich with the anchor and collagen sponge (see Figure 3A). The device is introduced with its own delivery sheath and is available in 6 and 8 Fr sizes. The 8 Fr Angio-Seal device has substantially more collagen in the sponge (28 g) than the 6 Fr device (18 g) and may offer an advantage in patients with antithrombotic and anti-platelet medications, regardless of the use of smaller sheath sizes for the procedure. A prospective registry including 1,004 patients undergoing both diagnostic and interventional coronary procedures using the latest generation novel automated Angio-Seal device demonstrated over 99 % deployment success for all procedures, with major vascular complications occurring in 0.4 % of these patients (0.2 % for diagnostic, 0.7 % for PCI).19 Although there are reports of successfully closing large-bore (>8 Fr) arteriotomies with more than one Angio-Seal using a ‘double wire’ technique, it is generally accepted that this device should be used for the 8 or 9 Fr or smaller sheath sizes.
The Perclose® suture-based devices (Abbott Vascular, Redwood City, CA) are the next most commonly used devices.20 The devices can additionally be used for pre-closure of large-bore arteriotomies with more than one device.21 Active approximation is achieved by sutures delivered across the anterior wall of the femoral arteries, with an arteriotomy site closure similar to that obtained via direct surgical cut-down (see Figure 3B). Success in achieving haemostasis with the Perclose devices exceeds 90 % with experienced operators.22 As with all other VCDs, success is highly dependent on the patient substrate and femoral arteries. Significant atherosclerosis and calcium deposits may result in a failed deployment of the sutures by redirecting the needles away from the intravascular footplate that must ‘catch’ them for the mechanism to work. Closing the suture over a wire is easily performed and offers a potential bail-out if the device fails. This device has evolved significantly since its original concept in 1994 (Prostar®), making it easier to use than the original device. The Prostar device is indicated for closure of up to 10 Fr sheaths, although it is now commonly used for sheaths >10 Fr. It remains essentially unchanged from its original design and requires hand-tying of the suture to secure the arteriotomy.
As previously mentioned, there are many new percutaneous procedures requiring large-bore equipment, such as thoracic endografts and aortic valve implants. Haemostasis in large arteriotomies requires a different approach from the routine and traditional techniques used for diagnostic and interventional coronary procedures. In this context, the concept of pre-closure has been an accepted alternative to surgical cut-down. Suture-mediated percutaneous closure has been described using the ‘pre-close’ technique in previous studies using both the Prostar XL and ProGlide Perclose devices (Abbott Vascular, Redwood City, CA). Multiple ProGlide devices (up to three), deployed at different angles prior to the procedure, with the monofilament suture being clamped and moved to the side, provide adequate haemostasis for most large-bore arteriotomy sites. This pre-closure technique is used during procedures requiring large-bore femoral arterial access and may preclude the need for surgical cut-down and closure, though common femoral puncture is imperative. Clinical experience suggests that there is preference for the ProGlide device because of ease of use compared with the more difficult to use, but larger, Prostar XL.23
The StarClose device (Abbott Vascular, Redwood City, CA) is a system that delivers a nitinol clip from the extravascular side to close the arteriotomy and achieve haemostasis. This device is used for closing 5–8 Fr holes and has a reported success rate over 90 % with experienced users (see Figure 3C).24 The StarClose is inserted over a wire through a sheath that comes with the device and is specially designed and required for use. A retractable anchor is used to ensure satisfactory positioning at the arteriotomy and the clip is then deployed on the exterior side of the artery. However, there have been reports of excessive oozing from the access site and there is the potential to inadvertently deploy the clip in the posterior wall, both anterior and posterior walls, or the overlying tissue.
When deployed, there should be no intraluminal component and this provides an attractive alternative to other closure devices for some operators.
New devices continue to be developed in an effort to find the optimal closure device: immediate haemostasis without leaving anything behind. These new devices use novel approaches to closure and are enjoying increased utilisation following their initial approval and commercialisation. The Mynx® VCD (AccessClosure, Inc., Mountain View, CA) is composed of an extravascular polyethylene glycol (PEG) sealant that is completely bioabsorbable (see Figure 3D).25 Haemostasis is achieved when an intra-arterial balloon approximates the PEG plug to the extravascular space anterior to the arteriotomy, providing temporary haemostasis. The PEG plug then hydrates with fluids from the access site to expand and compress the arteriotomy site externally. This device and the other passive extraluminal devices potentially provide effective haemostasis in patients with peripheral vascular disease, in contrast to active approximation devices, which are relatively contraindicated, although this hypothesis has not been formally tested. The EXOSEAL™ device (Cordis, Miami, FL) is a passive closure device that also delivers an extravascular plug to the arteriotomy site.26 It is also bioresorbable; its initial efficacy and safety appear to be satisfactory and it has recently been approved for use in the US. The Femoral Introducer Sheath and Hemostasis (FISH™) device (Morris Innovative, Bloomington, IN) incorporates a sleeve of small intestinal submucosa onto the sheath and provides haemostasis immediately after sheath removal when it is released into the arteriotomy site. Clinical experience with this device is extremely limited.27 The AXERA™ device (Arstasis, Redwood City, CA) facilitates haemostasis through the novel concept of creating an oblique arteriotomy within the arterial wall that augments manual compression when the sheath is removed.
Cost-effectiveness of Vascular Closure Devices
Despite improvements in technology and an increase in overall clinical experience, these devices are used in a minority of patients in the US. Multiple factors are responsible for this under-utilisation, despite data regarding their safety and efficacy. Importantly, concern regarding increased procedural costs without adequate reimbursement has dissuaded many interventionalists from incorporating closure devices into their practice. Two studies evaluated the cost-effectiveness of VCDs and observed a reduction in post-procedure costs, due primarily to a reduction in length of stay.28,29 In a more recent study comparing routine use of the Angio-Seal closure device and manual compression, routine use of the Angio-Seal device was associated with a US$44/case lower cost of a PCI procedure, driven primarily by a reduction in the rate of vascular complications.30 This cost saving was sensitive to the cost of manual compression as well as the incidence of vascular complications. With increased emphasis on the use of newer strategies to reduce bleeding complications, this cost saving may be reduced for low- or intermediate-bleeding-risk patients. Further investigation is needed to better understand the cost-effectiveness of closure devices in routine practice.
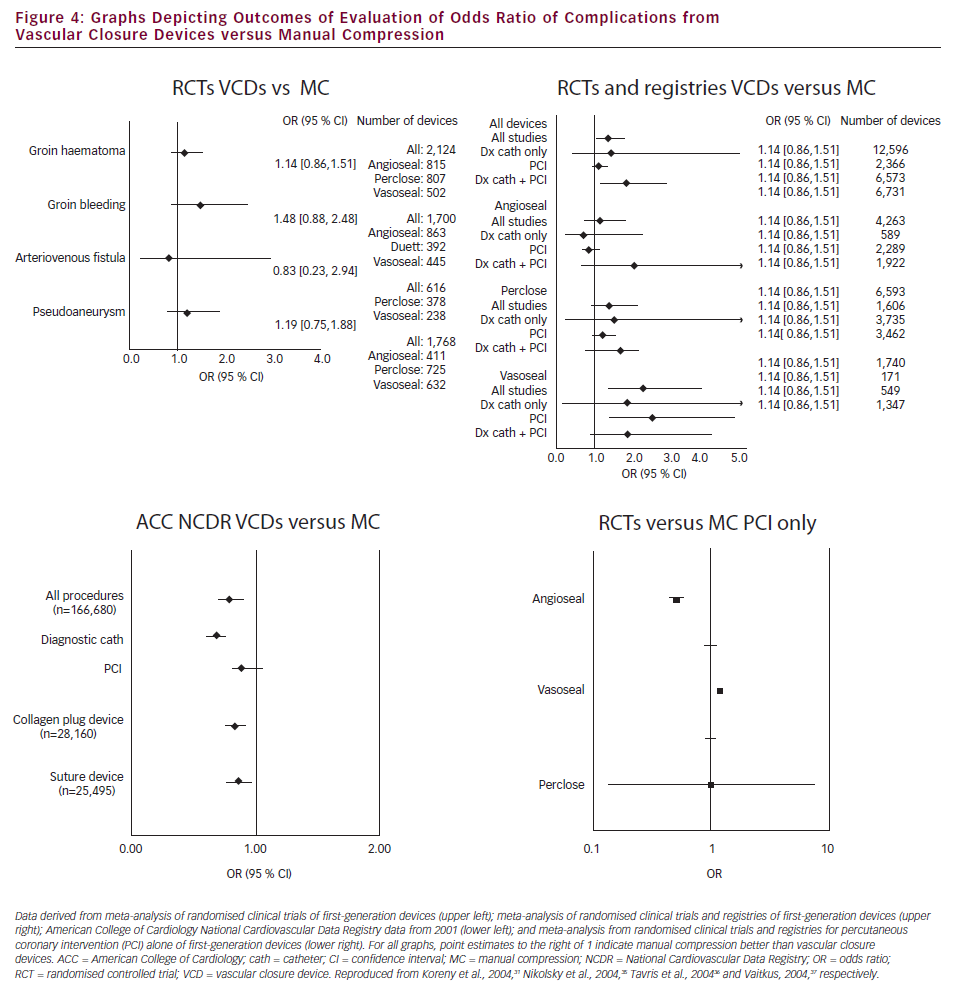
Clinical outcomes with Vascular Closure Devices Although VCDs continue to be used in a large number of patients worldwide, there remains a paucity of large randomised clinical trial data comparing the devices with manual compression. Many of the current data rely on meta-analysis of first-generation devices and the initial operator experiences with these devices (see Figure 4).31 Although there are currently no randomised trial data that indicate that VCDs lower the rate of vascular complications, in contemporary registries of greater than 1,000 patients comparing the complications of patients receiving manual compression or VCD the risk of vascular access complications with VCDs is consistently lower than with manual compression (see Table 4).32 Moreover, in an analysis from the American College of Cardiology (ACC) NCDR of approximately million patients who underwent PCI, VCDs were associated with a decrease in the risk of bleeding in patients with intermediate and high bleeding risk. Use of VCDs also provided a synergistic decrease in rates of vascular complications when used in conjunction with bivalirudin as compared with heparin.16 These data support the concept that the newer generations of closure devices are at least as safe as, if not safer than, manual compression.
Summary
The femoral approach continues to be the preferred site for the majority of operators in the US for catheterisation and interventional procedures.33 Femoral access remains a vital route for many procedures, including those involving the use of large delivery systems such as aortic valve implants and aortic endografts. Although the safety of diagnostic coronary catheterisation and interventional procedures performed from the femoral artery are well established, use of this access site is still a source of complications.34 Dissatisfaction with haemostasis achieved by manual compression resulting from patient discomfort, delayed ambulation and increased demands on cardiac catheterisation staff provides a fertile ground for the continued development of VCDs that afford quick and effective haemostasis after the sheath is removed. Despite shortening the time to haemostasis and ambulation, a debate still rages regarding their overall contribution to reducing complications and healthcare costs.