Transcatheter aortic valve implantation (TAVI) via femoral arterial access has become the treatment of choice for elderly patients with severe calcific aortic stenosis (AS) who are at intermediate or high risk for surgical aortic valve replacement (SAVR).1,2 More recently, randomised trials have led to the approval of TAVI for patients at lower surgical risk, thereby expanding its applicability to a larger cohort of relatively younger patients with AS.3,4 Over the past 10 years, technological advancements and design improvements have led to lower profile transcatheter heart valves (THVs), which allow easier and safer percutaneous delivery through smaller sized/diseased femoral arteries or calcified tortuous aortas, and also enable precise, predictable and safer deployment resulting in early ambulation and discharge.5–7 The vast majority of TAVI procedures worldwide are performed using either a balloon-expandable THV – commonly SAPIEN 3 (Edwards Lifesciences) – or a self-expanding THV – commonly Evolut R and Pro (Medtronic). These THV, now in their third generation of design iterations, were used in the pivotal randomised trials of TAVI versus SAVR.8 There are, however, newer THVs in varying stages of development or early clinical use, which aim to either improve on the two commonly used THVs or address the needs of difficult and unusual anatomies. In addition, the development of new generation THVs in India helps to make the device less expensive, thereby making this life-saving procedure accessible to more patients across the world – an important consideration in the context of the current economic constraints of healthcare delivery.
The Myval is a balloon-expandable THV developed by Meril Lifesciences, which has novel operator friendly design features to improve deliverability and aid precise deployment. Following the first-in-human MyVal-1 study, Myval was approved by the Central Drugs Standard Control Organization (CDSCO) of India in October 2018 and was CE marked in the European Economic Area in April 2019.9 It is presently approved for commercial use in 60 countries and more than 8,000 TAVI procedures using Myval had been performed globally by January 2023. Two pivotal randomised studies comparing Myval to the currently used THVs are ongoing.
Myval Transcatheter Heart Valve System
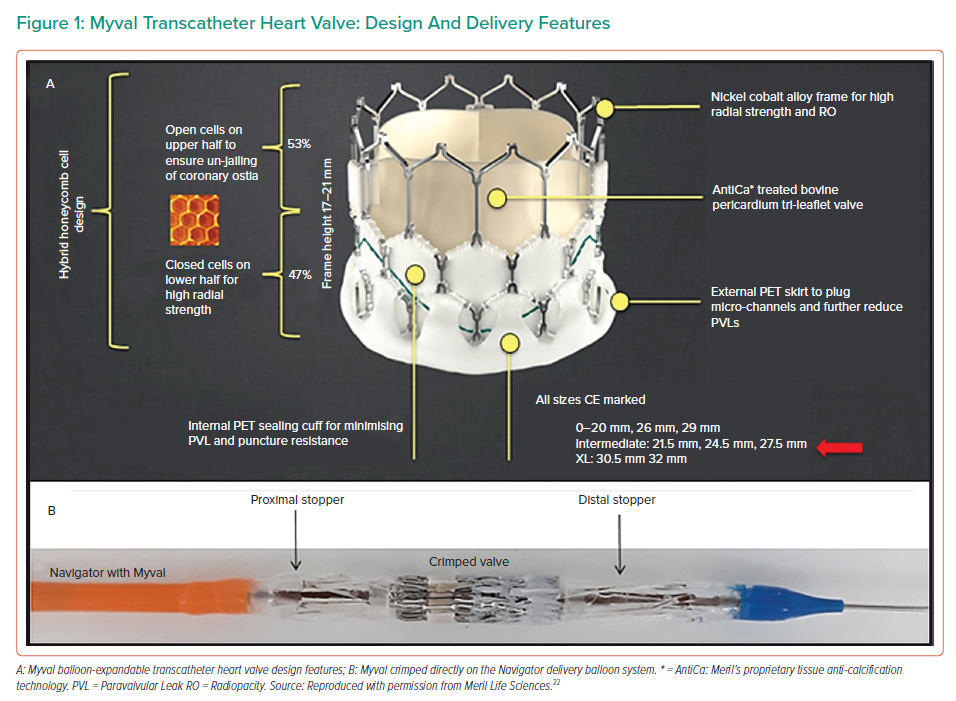
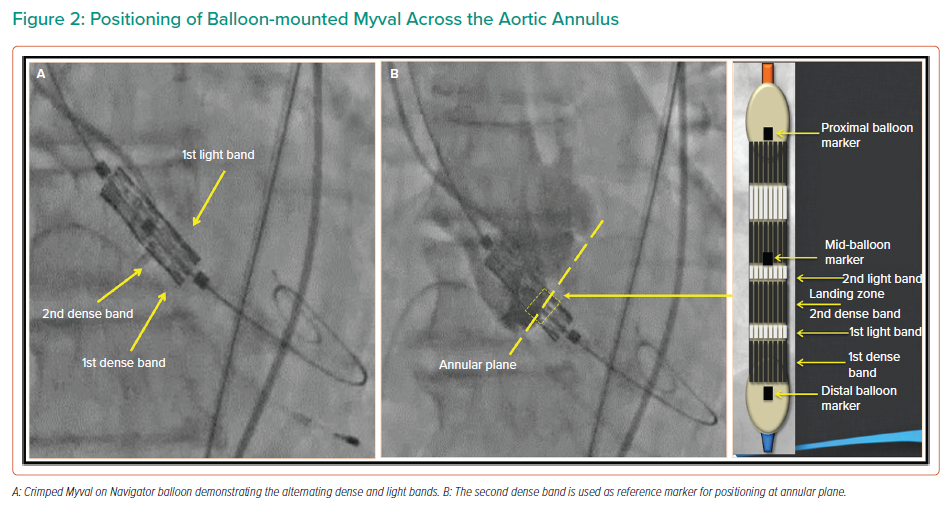
The Myval balloon-expandable THV system (Figure 1A) is made up of MP35N-Cobalt alloy framed hexagons. The hexagons are arranged in a hybrid honeycomb manner with 47% of the frame made up of closed cells at the ventricular end, providing a higher annular radial force, and 53% of the frame made up of open cells at the aortic end. On crimping, this design geometry results in an alternative dark/light band-like pattern which can be used for reference markers during positioning and deployment of the THV across the native annulus (Figures 1B and 2A). The lower ‘closed cell’ position of the valve frame is covered externally with a PET sealing cuff to minimise paravalvular leak. Myval is available in conventional sizes – 20 mm, 23 mm, 26 mm and 29 mm; intermediate sizes – 21.5 mm, 24.5 mm and 27.5 mm; and extra-large sizes – 30.5 mm and 32 mm. This enables clinicians to choose the most appropriate valve size for differing native annulus geometries.
The Myval is delivered by a specifically designed hi-flex, over-the-wire balloon-catheter delivery system: Navigator (Meril Lifesciences). The distal end of the Navigator delivery system can be flexed to more than 180° while navigating the aortic arch and crossing the native valve. At the distal end of the delivery system is a balloon with two counter-opposing soft stoppers (housed within the balloon) that create a shallow, low-profile zone where the Myval THV is crimped directly (like a stent mounted on a balloon) (Figure 1B). There is no covering sheath, so the stoppers prevent the valve from dislodging during delivery and this ‘deliver and implant’ feature makes the procedure more intuitive, simpler and safer. The balloon has proximal and distal internal inflation ports resulting in a dog-bone shape which stabilises the valve during inflation and deployment. All sizes of crimped Myval are inserted through an expandable hydrophilic Python introducer sheath (Meril Life Sciences) which has a 14 Fr internal diameter (ID) and expands momentarily to allow the passage of the pre-mounted THV. In case it has not been possible to cross the calcified aortic valve, the sheath design permits complete retrieval of the valve from the patient and reinsertion of the same valve.
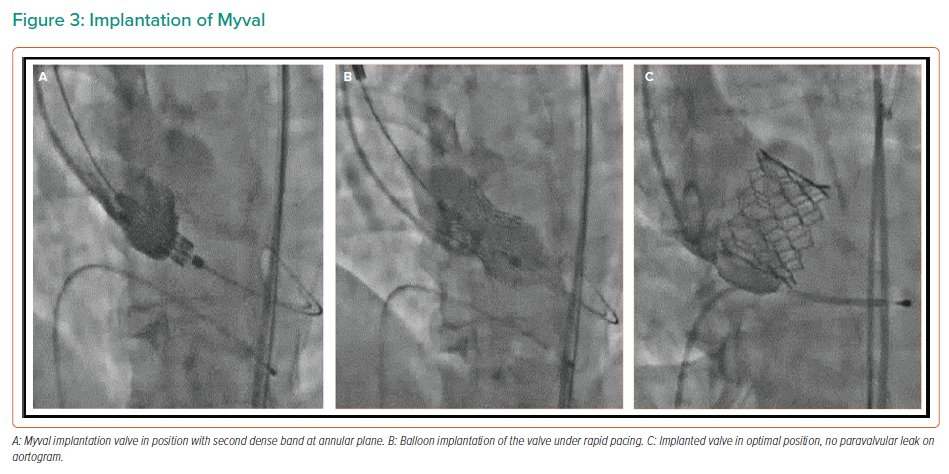
Balloon valvuloplasty of the native valve may be performed according to the operator’s discretion prior to Myval deployment. Thereafter, Myval is delivered across the native annulus, and precise positioning of the valve is achieved using the dark and light bands as reference markers, with the second distal dense band positioned at the annular plane (Figure 2B). This results in a post-implantation aortoventricular depth of 80:20 or 90:10 in relationship to the annulus. The Myval is implanted by inflating the balloon under rapid pacing as per balloon-expandable THVs (Figure 3).
Differences Between Balloon-expandable Myval and Sapien 3
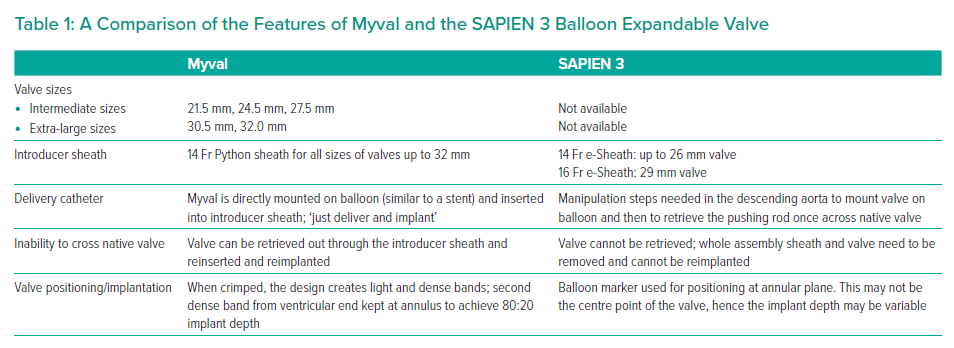
The Myval differs from Sapien 3 in a number of technical features which help to simplify the procedure and aid accurate valve sizing and implantation. These are listed in Table 1.
Pre-clinical Evaluation
The development work for Myval THV began in 2015. The device underwent fatigue testing that usually involves 200 million cycles (equivalent to 5 years of bioprosthetic life in a patient) as per International Organization for Standardization (ISO) 5840-1:2021 and ISO 5840-3:2021 guidelines. According to the latest guidelines, the valves have been tested up to 400 million cycles (10 years of use) and is currently at the 600 million cycles (15 years of use) testing phase. The current testing report is satisfactory. This was followed by animal studies where Myval THV was implanted in a sheep (ovine) model using the novel technique of external banding at ascending aorta to mimic calcification and deployment of Myval THV (either 20 or 21.5 mm) using a transcarotid TAVI approach. There was no incidence of device-related mortality. In all 11 surviving sheep, transthoracic echocardiography at 6 months demonstrated all implanted valves to be functional with no significant regurgitation, calcification, thrombi or vegetation. Healing was advanced in the sacrificed animals, with no instances of excessive cusp calcification. Histopathological analysis revealed full and stable integration and healing through endothelialisation and microcellular neointima without valve thrombosis. In addition to the animal studies, Meril also undertook acute ex vivo studies on human cadavers with severe AS to simulate TAVI procedure (Data on file, Meril Life Sciences).
MyVal-1 First-in-human Study
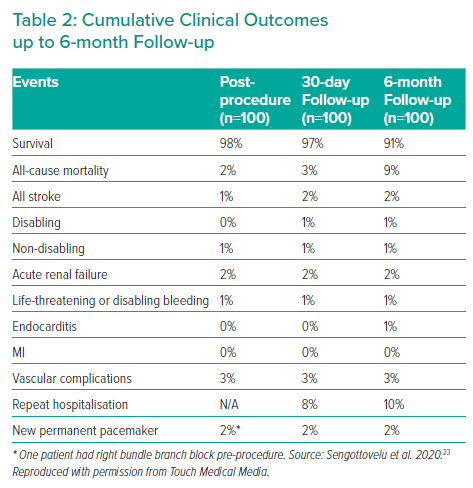
The MyVal-1 study enrolled its first patient on 30 June 2017.9 This prospective, multicentre, single-arm, open-label study evaluated safety and effectiveness of Myval THV in 30 Indian patients (as per the defined requirements of Indian regulatory authorities) with severe symptomatic native calcific tricuspid valve AS and ejection fraction ≥20% who were at intermediate- or high-risk for SAVR. The primary safety endpoint was survival at 12-month follow-up. The efficacy endpoints included improvement in New York Heart Association (NYHA) functional classification, effective orifice area (EOA) at echocardiography, 6-minute walk test from baseline, quality of life measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) and freedom from major adverse cardiac cerebrovascular and renal event at 12-month follow-up. Clinical echocardiography was performed post-procedure and at 1, 6 and 12 months and thereafter annually for up to 5 years. The mean age of patients was 75.5 ± 6.7 years and 73.3% of the patients were men. The mean Society of Thoracic Surgeon (STS) Risk Score was 6.4 ± 1.8 and 70% patients were in functional class III/IV. All procedures were performed through the femoral access route. Device success was 100%. Cumulative all-cause mortality was 3.3%, 6.7% and 13.3% at 1, 6 and 12 months, respectively. Two patients (6.7%) had major vascular complications. One patient suffered a non-disabling stroke. No patient required permanent pacemaker implantation. After the procedure, the mean aortic valve gradient decreased from 47.4 ± 8.8 mmHg to 8.0 ± 2.7 mmHg and the EOA increased from 0.6 ± 0.2 cm2 to 1.7 ± 0.3 cm2 and these improvements were maintained at 12 months. No patients had more than mild paravalvular leak. All patients were in NYHA class I/II and had a significantly improved quality of life and 6-minute walk test at 12 months. The MyVal-1 study was subsequently extended to 100 patients. The 6-month clinical outcomes of these 100 patients were presented at the PCR e-Course in May 2020 (Table 2).
Retrospective Single-arm Registries of Myval and Comparative Studies with Other Transcatheter Heart Valves
In a retrospective core lab analysis of quantitative video densitometric aortography, Kawashima et al. compared angiographic aortic regurgitation after TAVI among three balloon expandable valves – Myval (n=108), Sapien 3 (n=397) and XT (n=239).10 Myval THV had the least moderate/severe quantitative aortic regurgitation (2.8%) compared to Sapien 3 THV (8.3%) and Sapien XT THV (10.9%). The authors attribute this to the external skirt design minimising paravalvular leak, as well as the availability of intermediate and extra-large sizes, thereby doing away with the need for risky over- and undersizing and achieving a more accurate and optimal fit to the native annulus.
In a retrospective survey of 1,115 patients undergoing Myval implantation in 27 countries, Kawashima et al. also demonstrated that intermediate Myval sizing was used in 42% of patients in contemporary real-world practice, thereby addressing the unmet need of operators for a more calibrated THV sizing.11
Delgado-Arana et al. compared a matched population of 103 patients treated with Myval to 103 patients treated with Sapien 3.12 While the early safety and clinical efficacy were comparable at 30 days, there was a lower need for permanent pacemaker implantation (5.8% versus 15.5%, p=0.02) and significantly lower mean gradients and paravalvular leaks by blinded echocardiographic analysis in the Myval group compared to the Sapien 3 group. The investigators attribute this to the use of intermediate sizes of Myval in nearly 45% of the patients resulting in a possibly more optimal fit.
Santos-Martinez et al. reported the results of conduction disturbances analysed by a core lab in an academic European registry of 1,131 consecutive patients who underwent TAVI with any of the six THV – Myval, Sapien 3, Evolut, Accurate, Portico and Allegra.13 The Myval group had the lowest rate of permanent pacemaker implantation (7.4%).
García-Gómez et al. looked at 100 patients who were at low risk for SAVR and underwent TAVI using Myval in a retrospective study at nine European centres between September 2019 and February 2021.14 The mean age was 80 ± 6.5 years and mean STS was 2.4 ± 0.8%. The femoral access route was used in 98% and intermediate sizes of Myval were used in 39% of patients. Predilatation of the native aortic valve was performed in 43% of the patients. Procedural success was 99%. There were no cases of valve embolisation, annulus rupture, coronary occlusion, or procedural death. The permanent pacemaker implantation rate was 8%. Echocardiographic and functional improvement was maintained at 30 days and there were no deaths. There was moderate aortic regurgitation in 4% of patients.
In the recently published EVAL registry, Barki et al. performed a retrospective analysis of 166 consecutive patients undergoing TAVI with either Myval (n=58) or Evolut R THV (n=108).15 Early device success was significantly higher in the Myval group compared to Evolut R group (94.8% versus 83.3%, p=0.048). At 30 days and at 6 months, the Myval group had a significant lower incidence of moderate paravalvular leak (6.9% versus 19.8%, p=0.039) and permanent pacemaker implantation (11% versus 27.5%, p=0.02). The incidence of all-cause mortality and disabling stroke was similar in both groups.
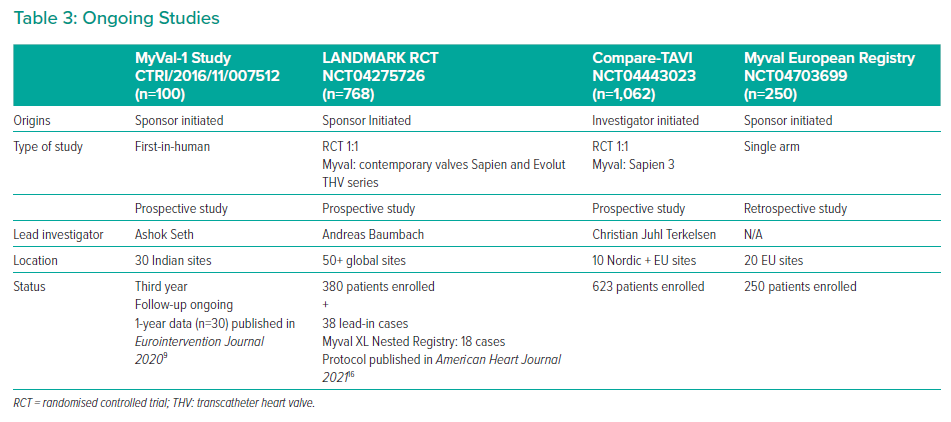
These favourable results of Myval, which have been attributed to its novel design and intermediate valve sizes are being investigated in the two ongoing prospective, large, randomised studies: the LANDMARK trial and the COMPARE-TAVI trial.
The LANDMARK Trial
The LANDMARK trial (NCT04275726) is a prospective, randomised, multinational, multicentre open-label non-inferiority trial ongoing in Europe, Latin America and South Africa with 768 patients randomised (1:1) to receive either Myval (n=384) or contemporary THV (n=384) – either from the Sapien THV series (n=196) or Evolut THV series (n=196). The primary endpoint is a 30-day composite of all-cause mortality, all strokes, prosthetic valve regurgitation, life-threatening or disabling bleeding, stage 2 or 3 acute kidney injury, vascular complications and conduction abnormality necessitating new permanent pacemaker implantation.16 The study population will undergo electrocardiographic and echocardiographic follow-ups annually until 5 and 10 years, respectively. Pre- and post-procedure aortic root angiogram will be used to evaluate the implantation depth and prosthetic valve regurgitation measured by video densitometry acquisition analysis. The trial was delayed due to the COVID-19 pandemic and the first patient was enrolled into the study on 26 November 2020. Up until 28 January 2023, 380 patients have been enrolled in addition to 38 lead-in cases. Further details including sample size and intended statistical analysis of this study have been published.16
Other Ongoing Studies
The ongoing studies involving Myval are listed in Table 3. Myval THV has been used with promising safety and efficacy for variety of indications in India and Europe including calcific bicuspid aortic valve stenosis, valve-in-valve for degenerated bio-prosthetic surgical valve in aortic and mitral position (although it is not yet CE marked for this indication) and in dysfunctional stenosed right ventricular tract conduits.17–19
Elkoumy et al. reported the retrospective analysis procedural and 30-day results of 68 patients who underwent Myval implantation in bicuspid aortic valve at 12 European centres.20 The mean age of patients was 72.6 and mean STS risk score 3.54. At the end of the procedure, the mean residual pressure gradient was 9.8 mmHg and EOA was 1.9 cm2. Only 3% cases had greater than mild aortic regurgitation. There were no procedural complications, no coronary obstruction, no aortic root injury, no device embolisation, no procedural death and no strokes. All-cause death was 3% and the permanent pacemaker implantation rate was 8.5%.
Other New Generation Balloon-expandable THV Under Investigation
The DurAVR (Anteris Technologies) is a novel new generation of balloon-expandable supra-annular THV made of 3D single-piece anti-calcification treated valve tissue which gives near-normal haemodynamics and extended durability. The valve design also enables commissural alignment for future coronary access. The first-in-human study with this valve is expected to start this year. PrizValve (Shanghai NewMed Medical) is a balloon-expandable valve being developed in China, which has undergone the first-in-human study and is planned to enter phase-2 studies this year (NCT05182307).21 The second gerneation Myval ‘Octacor’ THV with two identical rows of interlacing octagonal cells and additional features is currently undergoing human evaluation.
Conclusion
Myval represents a technological advancement over the previous generation of balloon-expandable THVs that makes the procedure simpler, safer and possibly more effective. Retrospective non-randomised comparative analysis and registries demonstrate favourable results and potential advantages of Myval over the current generation of THVs. Large prospective randomised studies are ongoing to evaluate its performance and long-term durability against commonly used self-expandable and balloon-expandable THV. If proven to be non-inferior, Myval could improve the availability and affordability of TAVI to a much larger population of patients across the world.