Patent foramen ovale (PFO) is a common abnormality, occurring in 20–34% of the population.1 In the majority of infants, closure of the foramen ovale occurs soon after birth, as negative intrathoracic pressure associated with the first breaths closes the PFO. In some cases, the primum and secundum atrial septa fail to fuse and closure remains incomplete. There is continuing communication between the right and left atria, particularly during actions that cause a sudden rise and fall in intrathoracic pressure, such as coughing, sneezing or straining. The changes can be mimicked by asking the patient to perform and then release a Valsalva manoeuvre.
For the majority of people, a PFO will remain undetected or appear only as a chance finding during cardiac investigation. However, some PFOs may open widely and provide a conduit for material such as thrombi, air or vasoactive peptides to travel from the venous to arterial circulation – a paradoxical embolus. This is associated with cryptogenic stroke, systemic embolus, migraine with aura, and decompression sickness in divers. Percutaneous PFO closure provides a practical and elegant solution to this problem in carefully selected individuals.
In this review, we evaluate the evidence for PFO closure, discuss which patients should be considered for this treatment and review how the procedure should be undertaken.
Patent Foramen Ovale: The Anatomy
During fetal development, the primum and secundum septa develop and overlap. This process occurs normally in patients with a PFO; the communication between the right and left atria that persists post-partum in patients with a PFO is caused by a failure of fusion of the two septa rather than a deficiency in either septum (Figure 1). This is distinct from a hole in either septum, which would constitute an atrial septal defect, a separate entity with different functional consequences and indications for closure (Table 1). However, both PFOs and atrial septal defects can permit the transit of a paradoxical embolism. In a PFO, the overlapping anatomy of the primum and secundum atrial septa forms a flap valve that usually only opens when the right atrial pressure exceeds the left atrial pressure. However, since right atrial pressure is usually less than the left atrial pressure, PFOs are functionally closed most of the time. However, this pressure gradient can be reversed by manoeuvres that change the intrathoracic pressure (e.g. coughing, sneezing or straining to defecate), thereby allowing the PFO to open and for blood, thrombus or any other substance to pass from the right to the left atrium.
Indications for Patent Foramen Ovale Closure in 2019
Cryptogenic Stroke
A cryptogenic stroke is one in which, despite extensive investigations, a clear cause cannot be found. This would include the exclusion of AF; atherosclerotic disease; carotid dissection; and intracerebral pathology, such as haemorrhage or space-occupying lesions.2,3 The cause of stroke remains unknown in up to 40% of patients with a stroke diagnosis. In PFO, the presumed cause of stroke is paradoxical embolus. Since the cause is known, the term is a misclassification but remains in use throughout the literature. Paradoxical embolus was first described by Zahn in 1881.4 The mechanism of stroke in PFO is translocation of venous thrombus to the arterial circulation under haemodynamic conditions where the PFO is opened. The opening of a PFO occurs during rapid fall and rise in right atrial pressure (e.g. after straining or coughing). Transient increase in right atrial pressure to greater than that of the left atrium opens a communication, and thrombus can transit at that brief moment. Several case studies demonstrating thrombus across a PFO support this mechanism,5–7 as do studies demonstrating the associations of venous thrombosis and PFO with cryptogenic stroke.8
Two early randomised controlled trials, Evaluation of the STARFlex® Septal Closure System in Patients With a Stroke or TIA Due to the Possible Passage of a Clot of Unknown Origin Through a PFO (CLOSURE I) and PFO and Cryptogenic Embolism (PC-Trial), did not demonstrate superiority of closure compared to medical therapy.9,10 These trials were confounded by a high crossover rate, failure to randomise those patients whose strokes were most likely to have been caused by PFO, limited power and the introduction of bias through inconsistent use of anticoagulants in the medical therapy group.11 Furthermore, the STARFlex occluder used in CLOSURE I was a poor device that has been abandoned in Europe owing to concerns about residual defects and left-sided thrombus formation.12
A number of recent randomised trials have demonstrated that PFO closure is superior to medical therapy. The early results of the Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) trial did not show benefit for PFO closure; however, recently, an extended follow-up of patients demonstrated that there was a reduction in ischaemic stroke compared with medical therapy (HR 0.55; 95% CI [0.31–0.999]; p=0.046; number needed to treat [NNT]=45).13,14 The Gore® Septal Occluder Device for PFO Closure in Stroke Patients (GORE REDUCE) trial demonstrated a significant reduction in clinical ischaemic stroke (1.45% versus 5.5%; p=0.002; NNT=25) compared with antiplatelet therapy alone.15 In the PFO Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) trial, no patients who underwent PFO closure experienced an ischaemic stroke, compared with 14 in the antiplatelet group (HR 0.03; 95% CI [0.00–0.26]; p<0.001; NNT=17).16 Finally, the Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High-Risk PFO (DEFENSE-PFO) study showed a reduction in the composite endpoint of stroke, vascular death and Thrombolysis In MI-defined major bleeding at 2 years with PFO closure compared with medical therapy (0% versus 12.9%; p=0.013; NNT=8).17 The results of these randomised controlled trials are summarised in Table 2.
Several meta-analyses have confirmed that PFO closure reduces the risk of ischaemic stroke in patients with cryptogenic stroke and PFO.18–20 These have shown that the overall absolute reduction in risk is low (1.0 per 100 patient-years), but this needs to be weighed against the long period of time that young patients are likely to be at risk. It is thought that patients with atrial septal aneurysm or large shunts may obtain greater benefit. Notably, in these trials and meta-analyses, AF was shown to occur more frequently after PFO closure than with medical therapy alone. This did not seem to counteract the overall stroke reduction in this population.
Participants enrolled in these trials were young, with most studies only including those under the age of 60 years. Participants were required to have symptoms consistent with a stroke, with confirmation of ischaemia/infarction on brain imaging. Confirmation of PFO with transoesophageal echocardiography (TOE) was also a requirement for enrolment. The studies excluded patients with an alternative attributable cause for their stroke (discussed in more detail below), and participants could be enrolled no more than 6–9 months after the index stroke. One of the major alternative explanations for embolic stroke is AF, and this was excluded in all patients.
These criteria are strict but, in the opinion of the authors, need to be respected in clinical practice since there is little or no evidence for treatment of PFO outside these criteria. Patients who meet these criteria should be considered for closure in preference to medical therapy.
Systemic Embolisation
Most paradoxical emboli are likely to present as ischaemic strokes, given the anatomy of the aortic arch. However, systemic embolisation to the gut, limbs and myocardium has been described.7,21–23 There is no evidence from randomised controlled trials that closure of PFO in the case of otherwise unexplained systemic embolisation is protective. Nonetheless, it seems logical that closure would be indicated in select cases. For example, closure of PFO would be indicated in a similar manner to that of cryptogenic stroke for a young patient presenting with ST-elevation MI of embolic source, normal coronary arteries and an absence of risk factors for atherosclerosis. Of course, care must be taken to exclude alternative explanations, and this may require optical coherence tomography to exclude in situ plaque rupture in the coronary artery. Given the myriad causes of myocardial injury, a cardiac MRI is recommended to confirm a pattern consistent with MI.
Decompression Illness
Decompression illness is a condition suffered by divers and high-altitude pilots who rapidly transition from high- to low-pressure environments. The sudden change in pressure results in formation of nitrogen bubbles within tissues that accumulate in the venous circulation. These are filtered from the bloodstream via pulmonary capillary diffusion. However, if return to low pressure (ascent from depth in the case of divers) is too rapid, then this pulmonary filtration process is overwhelmed and gas bubbles enter the systemic arterial circulation.24 These bubbles continue to enlarge and result in tissue trauma and even vessel occlusion. This can produce a range of symptoms, including muscle and joint pain, headache, dizziness, fatigue, rash, paraesthesia, breathing difficulties, confusion, motor incoordination and paralysis.
The presence of a right-to-left shunt such as a PFO allows nitrogen bubbles to bypass the pulmonary filter. Diving profiles are designed to limit the time at depth and slowly ascend toward the surface in order to minimise the risk of decompression sickness. The occurrence of a decompression illness despite such measures implies an increased risk of right-to-left shunt, and investigation for PFO should be considered.25,26 A longitudinal, non-randomised follow-up study showed a reduction in both symptomatic neurological events and total brain lesions among recreational divers with PFO and decompression illness who had PFO closure, compared with those continuing to dive without closure.27 In cases where a professional diver wishes to continue diving, a PFO closure could be recommended. The alternatives – stopping diving or curtailing provocative dive profiles – should also be considered. For recreational diving, the risk–benefit analysis for continued diving with a PFO closure is unclear, but some risk remains.
Platypnoea–orthodeoxia Syndrome
Platypnoea–orthodeoxia syndrome (POS) is a rare condition characterised by positional desaturation and dyspnoea in individuals with a PFO. Alteration of the geometry of the atrial septum allows continuous streaming of deoxygenated blood from the inferior vena cava across the PFO in certain body positions. Typically, the desaturation is seen with the patient seated, while oxygen saturations are normal when the patient is lying flat.28 Distortion of the atrial septal geometry may be caused by chest surgery, such as pneumonectomy, aortic dilatation and aortic surgery, or it may not have an identifiable cause. Occasionally, a tricuspid regurgitant jet can be directed across the PFO. POS is unrelated to underlying cavity pressures and responds well to PFO closure, provided that pulmonary artery pressure is not markedly elevated, which is usually not the case. A case series of 54 patients demonstrated that percutaneous closure could be achieved in a safe and effective manner.29
Migraine with Aura
Migraine is a common disorder in young people and is associated with aura in approximately a third of cases.30,31 Migraine with aura has been associated with right-to-left shunts, including PFO.32,33 Larger shunts have been found to be particularly associated with migraine with aura.34 The mechanism for the relationship between migraine and PFO is proposed to be the transfer of a vasoactive substance, usually filtered by the pulmonary circulation, into the systemic circulation.32
Non-randomised studies of PFO closure have reported improvement in patients’ symptoms after closure.35 The Migraine Intervention With STARFlex Technology (MIST) trial randomised patients with refractory migraine with aura to percutaneous PFO closure or a sham procedure.36 The trial showed no difference in cessation of headache or reduction in headache-free days. However, the trial assessed a population with a relatively low frequency of migraine, and there was a large number of residual shunts after closure. These problems may have negatively influenced the results. More recently, the Percutaneous Closure of PFO In Migraine With Aura (PRIMA) and Prospective, Randomized Investigation to Evaluate Incidence of Headache Reduction in Subjects With Migraine and PFO Using the AMPLATZER PFO Occluder to Medical Management (PREMIUM) trials have reported their results.37,38 Both studies were negative for their primary endpoints and, while there were some reductions in headache, the effects were small and occurred at the expense of procedural complications.
Overall, there is not enough evidence for PFO closure at present to offer a routine recommendation for therapy for this indication. PFO closure may rarely be considered in carefully selected individuals through a neurology multidisciplinary team, provided there is appropriate consent for procedural risk and with an understanding that an improvement in symptoms would not be certain.
The Patent Foramen Ovale Closure Procedure
Pre-Procedure Investigations
Since the most common indication for closure is cryptogenic stroke, an emphasis should be placed on work-up for other potential causes of stroke. Brain imaging should be undertaken to confirm the diagnosis of a stroke of embolic topography. Lacunar strokes are not likely to be embolic in nature. Carotid imaging should be undertaken to exclude significant plaque disease. Thrombophilia screening should be considered but is complex, with results that are sometimes inconsistent and often with a need for repeated investigations. Many thrombophilias predispose to venous more than arterial thrombosis, making interpretation of the results difficult, and this should be done in conjunction with haematologists with an interest in thrombosis.
AF is the most common source of thrombus, with studies suggesting that 13% of patients with AF have cardiac thrombus.39 Among patients with non-valvular AF, the thrombus was located in the left atrial appendage in 90%.39 The presence of AF in the context of a stroke is an indication for anticoagulation, and closure of a PFO is not indicated. No study has shown that closure of a PFO confers additional benefit. ECG monitoring is mandatory to exclude AF, and the duration depends upon the patient’s risk factors. In young patients (<50 years) with no risk factors, we recommend using a minimum of 72-hour ambulatory surface ECG recording, and in those aged >50 years we recommend using 6 months of implantable loop recording (ILR). ILR has the advantage of extended rhythm surveillance; however, it is prone to false positives and false negatives.40–42 Conclusive evidence for the best strategy to diagnose AF is lacking.
Transthoracic echocardiography (TTE) is the key first-line investigation for the exclusion of intracardiac thrombus. Cardiac thrombus is associated with a number of conditions apart from AF, including MI, atrial myxoma, left ventricular aneurysm, non-compaction cardiomyopathy, left ventricular failure and mitral stenosis. All of these need to be excluded prior to consideration of closure of PFO.
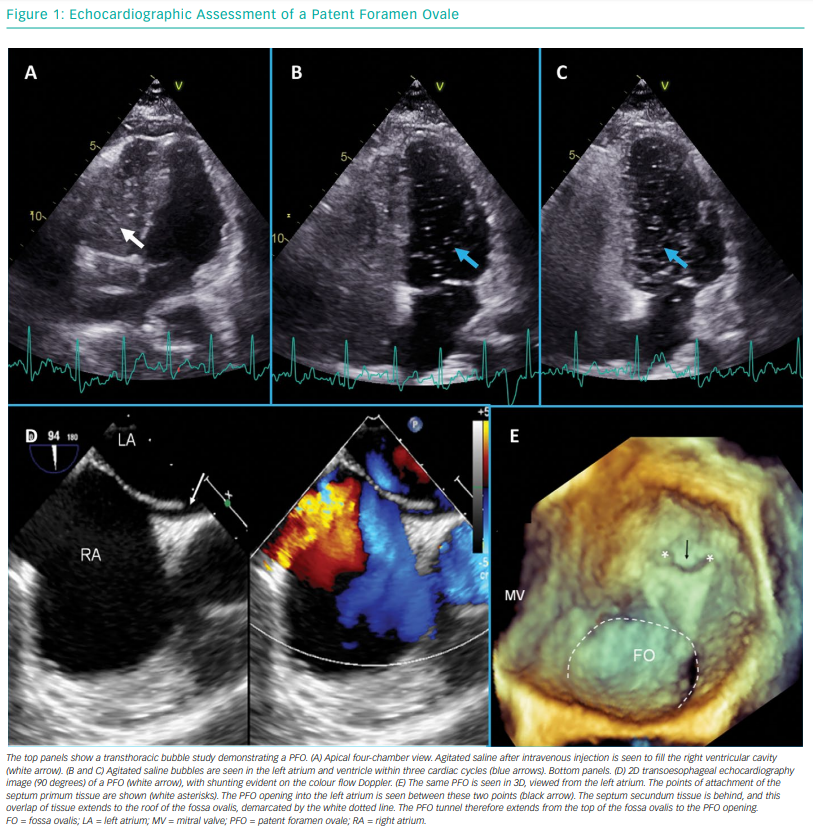
Bubble contrast echocardiography is a key investigation when working up patients with cryptogenic stroke. In order for a PFO to cause a stroke, it needs the ability to produce a right-to-left shunt. Bubble contrast studies are initially performed using TTE, with no sedation necessary. Agitated saline is injected into a peripheral venous cannula (ideally in the left antecubital fossa), and the patient is asked to perform a Valsalva manoeuvre or to sniff. In the presence of a cardiac shunt, bubbles should appear in the left side within three to four cardiac cycles of arrival in the right atrium. Late appearance of bubbles may reflect pulmonary transit, and performance by an experienced operator is needed. The procedure may require multiple repeats to confirm the diagnosis. Figure 1 shows a bubble study with transmission of bubbles from right to left.
A positive bubble study in the setting of cryptogenic stroke is an indication for detailed TOE. This allows the structural team to accurately define the position and anatomy of a PFO. TOE assessment of a PFO is also shown in Figure 1. The study will also exclude the presence of alternative shunts, such as ventricular septal defects, anomalous pulmonary venous drainage and sinus venosus defects. A full description of the TOE assessment of PFO is beyond the scope of this article but is reviewed elsewhere.43
The diagnosis of cryptogenic stroke and PFO will require the input of multiple specialties, including stroke physicians or neurologists, cardiac imaging specialists, radiologists and interventional cardiologists. Some centres use the Risk of Paradoxical Embolism (RoPE) score to help multidisciplinary teams classify the relationship between the stroke and the PFO.44 Consideration of the investigations and the patient as a whole should be undertaken in a multidisciplinary setting.
The Closure Procedure
PFO closure is routinely performed as a day-case procedure. The procedure can be performed in a standard catheter laboratory with fluoroscopic guidance and physiological monitoring. Since patients undergoing this procedure will obtain no immediate symptomatic benefit, with only the long-term risk of stroke being reduced, the authors of this review emphasise that all possible steps to reduce complications should be taken: the procedure should be, as far as possible, complication-free. In particular, this means using ultrasound-guided femoral venous access, echocardiographic guidance, adequate anticoagulation and special care to reduce risk of air embolus.
In the opinion of the authors, periprocedural guidance with TOE or intracardiac echocardiography is mandatory to consistently achieve the best result. General anaesthesia is generally required to facilitate TOE. The procedure is undertaken from the femoral vein, preferably using ultrasound guidance. Adequate anticoagulation should be administered (unfractionated heparin 80–100 IU/kg).
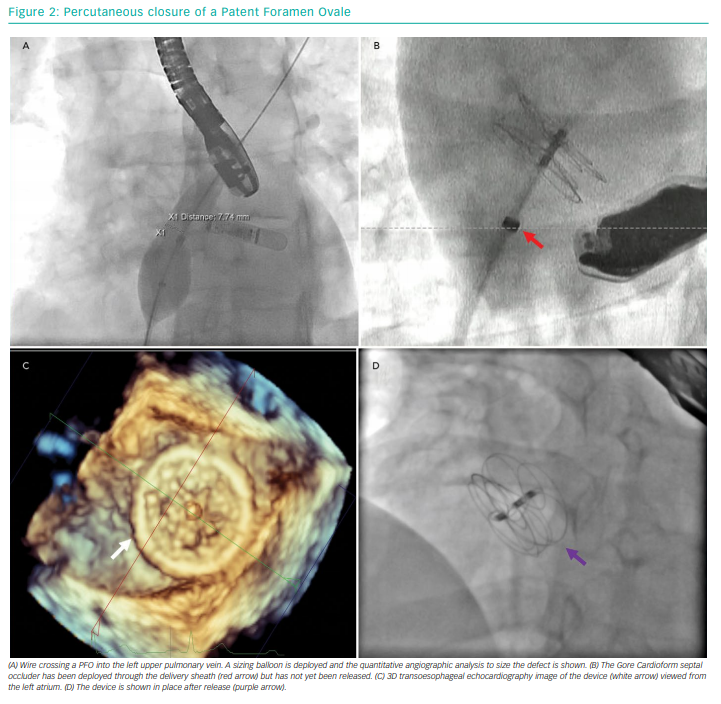
The PFO is crossed with a 6 Fr multipurpose diagnostic catheter. A 0.035 inch J-tipped guide wire is passed into a pulmonary vein (usually the left upper). This may be exchanged for a stiff wire to assist delivery of balloons. Balloon sizing of the PFO can be performed using quantitative angiographic tools. A left anterior oblique fluoroscopic projection may assist with this, as the septum is seen in profile. Compliant balloons with marked graduations are used, but balloon sizing can still shorten and widen the PFO. This may be desirable if there is a particularly long PFO tunnel, but it can enlarge the hole, thus necessitating a larger device. Similar (and potentially more accurate) information can be obtained through TOE assessment.
After sizing, an appropriate device can be selected and its delivery sheath introduced into the left atrium. The left atrial disc is deployed, followed by the right disc. Throughout this procedure, ensuring that the delivery sheath remains de-aired and flushed is crucial to minimise the risk of air or thrombotic embolism. Once the device is placed, confirmation of adequate positioning with echocardiography and fluoroscopy should be performed prior to device release. If the device is found to malpositioned after release, it can still be recovered using a large gooseneck snare. Figure 2 shows the steps involved in a PFO closure procedure.
The optimal regimen of antithrombotic therapy after device deployment remains uncertain. Aspirin and clopidogrel are usually given for 6 months in our practice, but evidence for this is limited and practice has varied markedly between trials. Some operators preload patients with antiplatelets, but again the evidence for this is uncertain. Single antiplatelet therapy, usually clopidogrel 75 mg daily, is continued indefinitely.
The patient should undergo TTE prior to discharge and at 6 weeks to exclude pericardial effusion and device embolisation. Closure rates are high with modern devices, and the principal objective is to stop the PFO flap valve opening wide, which occurs as soon as the device is deployed. Complete closure depends upon endothelialisation of the device and can take up to 6 months, after which time a repeat bubble study can be undertaken to confirm complete closure, although this is not mandated unless the patient plans to dive.
Closure Devices
A large number of devices with varying shapes and sizes have been marketed, with many achieving CE mark status in the EU. In the US, the need for evidence from randomised controlled trials prior to approval means fewer devices have been approved by the Food and Drug Administration.
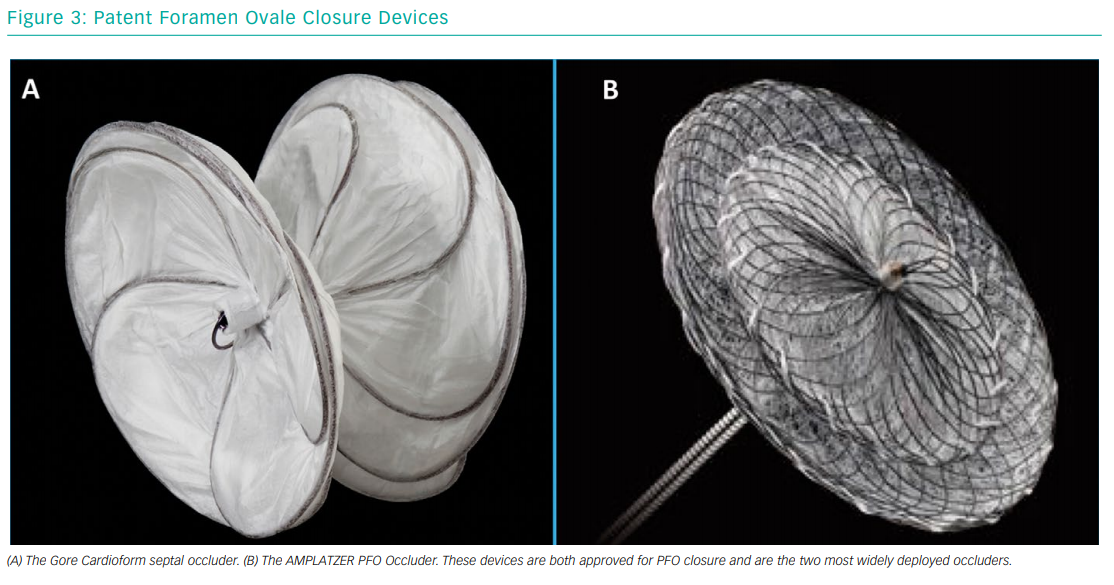
Most devices are of double-disc design, connected by a short waist. The Gore Septal Occluder (WL Gore & Associates) and the AMPLATZER PFO Occluder (Abbott Vascular) are two of the more commonly used devices (Figure 3). The Gore Septal Occluder is constructed from five nitinol wires covered with expanded polytetrafluoroethylene.45 Early clinical experience has shown that it is a versatile device with easy deployment, high procedural success rates and low complication rates.46,47 The AMPLATZER PFO Occluder is also a nitinol-based device. It is the device that has been most commonly used in randomised controlled trials, and the evidence for its use is, therefore, very strong.16,17 Operators should gain experience using different devices in order to give the best possible result for the patient.
Conclusion
In this brief review, the main indications for PFO closure (cryptogenic stroke, paradoxical systemic embolisation, POS and decompression illness) have been discussed, together with the strengthening evidence for closure. The skills needed for this procedure need to be learnt with the assistance of an experienced interventional cardiologist, who can proctor and advise those starting out with PFO closure. An attention to detail in the indication for the procedure and minimising the risks to the patient during closure are the key to an effective PFO closure service in 2019 and beyond.