Post-infarction ventricular septal defect (PIVSD) is a rare mechanical complication of acute MI (AMI) associated with very high acute mortality. It is a rupture between the left and right ventricle caused by infarction and necrosis of the muscular intraventricular septum. The incidence of PIVSD is approximately 0.2% in the era of primary percutaneous coronary intervention, but mortality has not improved despite advances in emergency revascularisation.1,2 Closure of the PIVSD defect was first performed surgically by Cooley et al. in 1957, and percutaneously by Lock et al. in 1988.3,4 It reduces mortality compared with historical data on survival with medical management alone. The evidence to support this is largely observational and retrospective. No randomised data exist for treatment of PIVSD. Published data and opinion regarding PIVSD must be interpreted with caution – observational case series are often limited in volume, and subject to both observer and selection bias. The rare nature of this complication’s occurrence combined with high procedural mortality limits systematic attempts to identify cases for high-quality observational studies. There is no consensus on many aspects of PIVSD treatment, including preprocedural optimisation and timing of closure. Until recently, there were no head-to-head data comparing surgical and percutaneous closure, but a retrospective analysis addressing both closure methods was published in 2022.5
Pathophysiology
PIVSD occurs as a result of acute rupture in the muscular portion of the ventricular septum following AMI. Known risk factors for PIVSD include female sex, older age, first MI, anterior infarct and hypertension.6 Chronic angina, diabetes and previous MI appear to be protective factors, theorised to reduce risk via ischaemic preconditioning and collateral protection.7
The anatomical location of the rupture is dependent upon the arterial territory affected, occurring with similar frequency in anterior and non-anterior locations.8 Anterior infarction is more likely to cause a defect in the apical septum, while non-anterior infarction is more likely to cause a defect at the basal septum, which can also involve the posterior wall, adding to the complexity of closure.9
Earlier studies suggested that PIVSD occurs approximately 1 day after AMI, with others suggesting a bimodal incidence, with peaks at 1 and 3–5 days.1,10 Defects can be characterised as simple or complex based on their course and relationship to surrounding structures. Simpler defects may have a direct communication between the left ventricle (LV) and right ventricle that is entirely intraseptal. Complex defects can take a serpiginous course between the ventricles and may not be confined to the septum.
Defect size can range from millimetres to several centimetres. The size and shape of the defect may vary with the cardiac cycle and can paradoxically be larger during systole, when the shunt volume increases, and the muscular tissue of the septum is non-contractile.11
An acute interventricular communication following rupture adds further physiological stress on a background of large territory infarct, by exposing the pulmonary vasculature to systemic pressure, with subsequent pulmonary hypertension and LV volume overload. LV volume overload will result in dilatation and reduced contractile function once Starling mechanisms are exhausted. Compensatory systemic vasoconstriction to maintain blood pressure will increase LV afterload, further worsening the left-to-right shunt and causing further decline. The degree of shunting is dependent upon cardiac contractility, defect size and the ratio of pulmonary:systemic vascular resistance. LV afterload is a key target for optimisation; factors that increase LV afterload or decrease right ventricle afterload will increase the left-to-right shunt; factors that decrease LV afterload or increase right ventricle afterload will reduce or even reverse the left-to-right shunt.12
Diagnosis
PIVSD is often diagnosed at the time of presentation with AMI or in the days after admission. The severity of clinical presentation or an acute deterioration with signs of decompensated heart failure and/or cardiogenic shock should alert the physician to carefully examine for PIVSD and perform immediate transthoracic echocardiography. Heart failure is often right-sided on clinical examination, and the patient may tolerate lying in the supine position, as pulmonary oedema is often absent.13,14 Alternatively, in a small defect, the injury may be found ‘incidentally’ (before it manifests physiologically) through auscultation of a new pan-systolic murmur, almost invariably present in PIVSD, and which may be accompanied by a thrill in up to 50% of patients.13 In the presence of low cardiac output states, however, precordial findings may be difficult to detect.
Invasive diagnostic methods, such as right heart catheterisation, are infrequently performed in the modern era, as non-invasive methods yield sufficient information, mitigating the risk associated with an invasive procedure. Left ventriculography in the left anterior oblique cranial view can reveal a left-to-right cardiac shunt. Right heart catheterisation will show a significant step up in oxygen saturations from the right atrium to right ventricle. Tricuspid regurgitation may occur due to right heart failure, and care must be taken not to sample a tricuspid regurgitant jet when measuring right atrial saturations. The severity of pulmonary hypertension is variable and influenced by clinical factors, including time passed since PIVSD formation. Pressure waveform interpretation in PIVSD is non-specific, but may reflect ventricular status, and any associated tricuspid or mitral regurgitation.
Transthoracic echocardiography is the main diagnostic modality for PIVSD, providing anatomical location and defect size by identifying focal disruption of the interventricular septum with evidence of left-to-right shunt by colour Doppler. Transoesophageal echocardiography (TOE) may augment the diagnostic yield pre- and intraoperatively, particularly if surface windows are suboptimal. Transthoracic echocardiography will often underestimate the size of the defect, as the ultrasonic beam is not coaxial with the defect. 3D echocardiography can add superior qualitative and quantitative assessment of the defect in comparison with 2D echocardiography.15
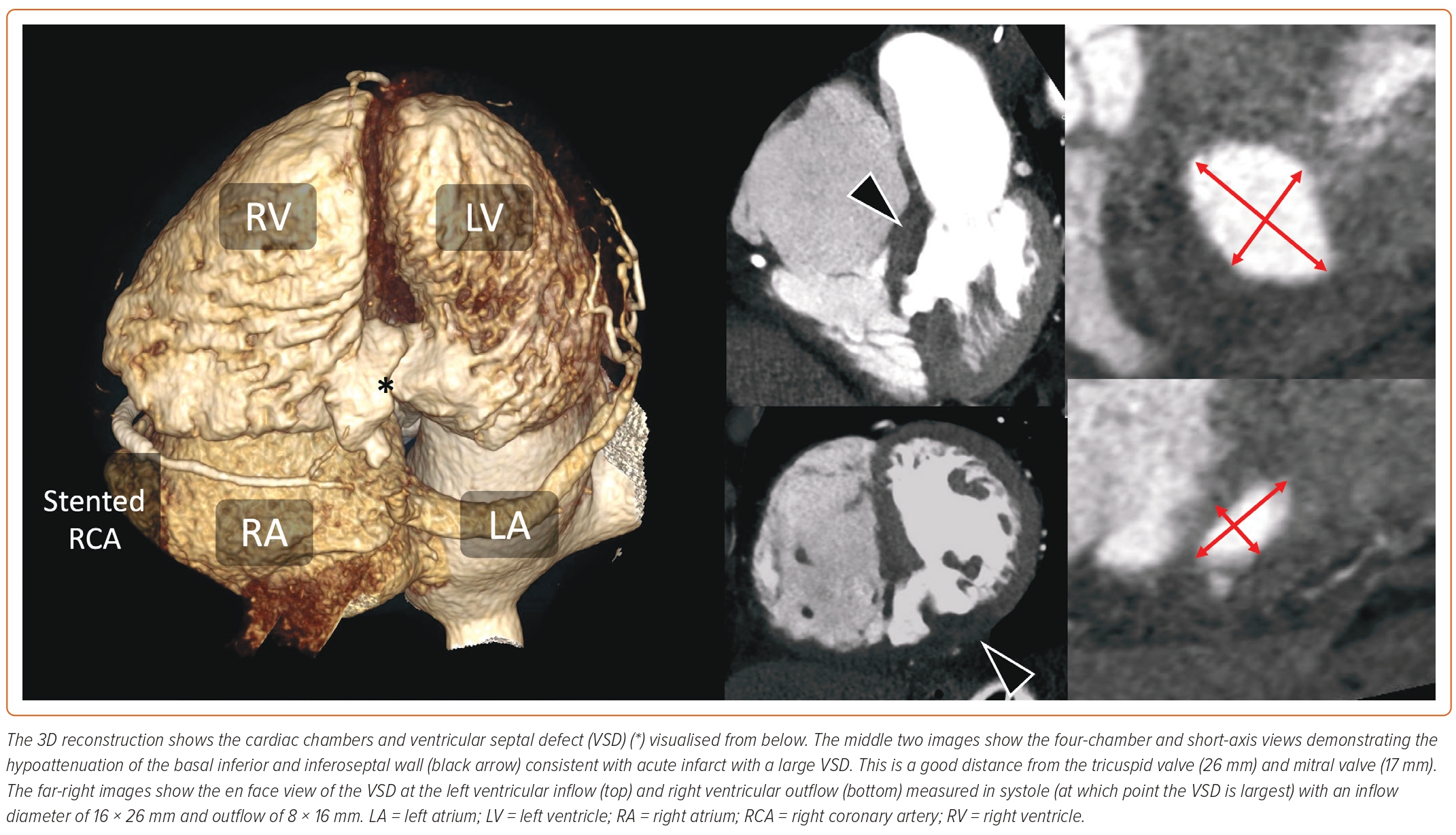
While echocardiography is more commonly used to assess PIVSD, CT, with its high spatial and temporal resolutions, multiplanar reconstruction capabilities, and wide field of view, may provide valuable adjunctive information. As such, cardiac CT is increasingly being used for the assessment and characterisation of septal defects – providing detailed information on rim thickness and margins, as well as the proximity of the defect to other heart structures.16 The role of cardiac CT is shown in Figure 1. Cardiac MRI is well suited to assessment of PIVSD, providing both anatomical and functional information, including shunt volume. It can also provide assessment of PIVSD size and characteristics, as well as viability assessment of bystander disease territories. However, patients may be unwilling or unable to tolerate the study, and long breath hold requirements may not be feasible in the critically unwell. CT has the advantage of faster scan times in more unstable patients, and the ability to scan patients with hardware-specific contraindications for MRI, such as mechanical circulatory support devices.
Serial interrogation of the defect can produce variable size measurements due to necrotic extension or contraction, and it is important to factor this into preprocedural planning and equipment procurement.
Evidence for Closure
Historical data suggest medical management alone without any form of closure carries a mortality rate of 94% at 30 days.1 No randomised evidence for the treatment of PIVSD has been published to date. The role of surgical closure as the gold standard rests in historical norms and opinion. A benchmark surgical study is an analysis of Society of Thoracic Surgeons data, including 2,876 patients operated for PIVSD between 1999 and 2010. This showed operative mortality (death from any cause either in-hospital or within 30 days of the index operation) of 42.9%.17 Percutaneous closure series are smaller in volume and heterogenous in their reporting. In 2016, Schlotter et al. undertook a systematic review of all published observational series involving percutaneous transcatheter closure, showing in-hospital/30-day mortality was 32% among 273 patients.18 Mortality ranged from 18% to 75% in individual series.
The first substantive examination of both surgical and transcatheter PIVSD closure was recently undertaken by Giblett et al. in 2022.5 This study involved a retrospective case note review of 362 patients undergoing 416 interventions (131 percutaneous, 231 surgery) in 16 UK centres between 2010 and 2021 (after the establishment of the primary percutaneous coronary intervention (programme nationally). Procedural success was 79.4% in percutaneous closure and 88.3% in surgical closure. Patients undergoing percutaneous closure were older, and more likely to have had their case discussed at a Heart Team meeting. Patients undergoing surgical closure had a higher likelihood of being recorded as having cardiogenic shock and receiving mechanical circulatory support placed prior to intervention. The median time from AMI to presentation was 2 days, with a further 7 days from presentation to definitive treatment. In-hospital mortality for all patients was 48.1%, higher in the percutaneous cohort (55.0% versus 44.2%, p=0.048). Five-year all-cause mortality was similar between both groups. Multivariate analysis showed that independent factors associated with both in-hospital and long-term mortality included cardiogenic shock, earlier time to intervention, an initial percutaneous closure strategy and baseline creatinine. Percutaneous coronary intervention to the infarct related artery was associated with in-hospital mortality, while the extent of bystander coronary artery disease impacts long-term mortality.
Concomitant surgical revascularisation in those with bystander coronary artery disease is not mandated, but a trend towards improved survival after revascularisation has been demonstrated.19 The European System for Cardiac Operative Risk Evaluation II can be used as a predictor for operative mortality in surgical closure.20 The Model for End-Stage Liver Disease Excluding Internationalised ratio score has also been used as a predictor for 30-day mortality in those undergoing percutaneous closure, and shown to have high sensitivity and specificity.21
Surgical and Percutaneous Closure: Comparisons
Comparing outcomes between surgical and percutaneous closure remains difficult, given the small volume of PIVSD interventions carried out per year, a paucity of substantial head-to-head comparative data and the cross-over that occurs between modalities. A total of 16.1% of patients in the percutaneous arm of the UK registry required additional surgical intervention, and 7.8% in the surgical arm required additional percutaneous intervention.5 There is substantial selection bias, with those undergoing surgical closure being younger and more likely to have been in cardiogenic shock. Many centres reserve percutaneous closure attempts only for those not accepted for surgery. Giblett et al. suggested similar long-term mortality outcomes between both options. A landmark analysis of survival after hospital discharge showed no difference between techniques. There was a small, but significant, difference in in-hospital mortality, higher in the percutaneous group. This is despite higher rates of stroke, renal replacement therapy and pneumonia occurring in the surgical cohort. The cohort undergoing percutaneous closure attempts may have a bimodal distribution between the well/simple defect and the unwell/complex defect deemed unsuitable for surgery.
There are several absent and confounding factors that make interpretation of observational data difficult in PIVSD. These include frailty and absence of reporting on those treated palliatively or who did not survive to attempted closure. Age highlights confounding variables, as it was not independently associated with mortality, but was associated with a percutaneous approach, which was itself associated with increased in-hospital mortality. A surgical approach in the first instance has often been favoured, due to lack of experience with transcatheter closure. Some physicians have suggested immediate transcatheter device closure over surgical closure or delayed closure.22 Both treatment modalities appear durable after discharge, suggested by their similar long-term mortality outcomes. In the absence of randomised data, it is the authors’ opinion that decisions regarding closure technique should be individualised and guided by Heart Team decision-making.
Timing and Preoperative Care
Consensus opinion regarding optimal timing for PIVSD closure is open to interpretation in the absence of high-level evidence. The European Society of Cardiology guidelines suggest early surgery should be performed for all patients with severe heart failure not responding rapidly to aggressive therapy, but delayed elective closure may be considered if patients respond to stabilising measures.23 The American Heart Association/American College of Cardiology guidelines suggest emergency repair is necessary, even in haemodynamically stable patients, to avoid abrupt deterioration.24 However, a more recent scientific statement from the American Heart Association is more in line with the European Society of Cardiology guideline.25 Neither the US nor European guidelines define the length of time.
Registries and case series repeatedly provide data that suggest that delayed closure improves survival in both surgical and percutaneous treatment. These studies are subject to substantial survival bias and immortal time bias, as they exclude patients who die while waiting for treatment, and they often include patients who have deteriorated after a short delay, when they would have represented a lower risk going to surgery earlier. This is sometimes described as a ‘trial of survival’. Delay for stabilisation, when appropriate, may facilitate organisation of friable defect tissue and enhance the chance of successful closure, but at the risk of encountering abrupt deterioration in clinical status if the defect extends itself further during this wait. Ultimately, the optimal timing remains subjective and opinion-led in the absence of prospective, and better randomised data, to guide decision-making.
Non-closure management of PIVSD is temporising only, given the mortality associated with medical management alone, and should be viewed as a bridge to defect closure or advanced heart therapies/transplant, unless palliative measures are put in place. The aim of preclosure management should be to maintain end-organ perfusion with optimisation of cardiac output, and reduction in afterload and pulmonary pressures. Pharmacotherapy requires a tailored approach with vasodilators, vasopressors, inotropes and diuretics. Vasodilators are intended to reduce LV afterload, in turn reducing left-to-right shunt through the defect. PIVSD-associated hypotension often precludes use of vasodilatory and diuretic therapies, however. Vasopressors increase systemic vascular resistance and, in turn, blood pressure and end-organ perfusion. They must be used cautiously, as this increase in systemic vascular resistance will increase myocardial oxygen consumption, afterload and the degree of shunting, with a subsequent potential reduction in cardiac output. Inotropes will increase cardiac output at the expense of increased myocardial oxygen consumption and shunting.
Mechanical circulatory support (MCS) may allow haemodynamic stabilisation to facilitate closure, although the optimal device is not yet known and there is no evidence that it independently improves long-term mortality outcomes. Intra-aortic balloon pump (IABP) counterpulsation has been in use since the 1970s and is the most common type of MCS used in PIVSD.26 It mildly increases cardiac output while reducing afterload and shunt volume, as well as improving coronary perfusion. IABP cost and complication rates are lower than other forms of MCS, but its ability to augment haemodynamics is less. The European Society of Cardiology guidelines state that IABP counterpulsation may be considered for haemodynamic support in PIVSD to stabilise patients until defect closure can be performed.23
More advanced MCS has been reported, but used in lower numbers. These include venoarterial extracorporeal membrane oxygenation (VA-ECMO), Impella, TandemHeart and tailored combinations of these. Computer simulation modelling did not demonstrate haemodynamic normalisation with any of these devices, nor could any achieve a pulmonary-to-systemic flow ratio <2.0, although Impella appeared to be the best choice.27
Ronco et al. performed a systematic review of MCS use in PIVSD with 129 (5.3%) patients of a cohort spanning 20 years (2000–2020) receiving advanced forms of MCS. Almost all patients had IABP support. VA-ECMO was the most commonly used advanced MCS (77.5%), and in-hospital mortality was lower in the advanced MCS group, being lowest in VA-ECMO at 29.2%, 35.3% in Impella and 30.8% in TandemHeart, compared with 52% in the IABP cohort.26 The haemodynamic effect of VA-ECMO may negatively impact the PIVSD, since it increases LV afterload and therefore left-to-right shunting. However, it does maintain optimal end-organ perfusion and adequate gas exchange. Concomitant use of IABP or Impella may reduce this ECMO-associated LV loading and shunt effect.
Closure Technique
The surgical techniques for closure are well described elsewhere and beyond the scope of this review. Percutaneous techniques continue to be developed and evolve. The key to successful treatment is patient selection and preparation, combined with appropriate experience. Within any given country or territory, there are few operators with substantial experience and, therefore, early involvement of experts and a flexible approach to the technique are important. Transfer of the patient to experienced centres may be considered where the patient is stable, or the operator may need to travel to the patient. Significant delay to the availability of an experienced percutaneous operator should prompt further consideration of urgent surgical repair.
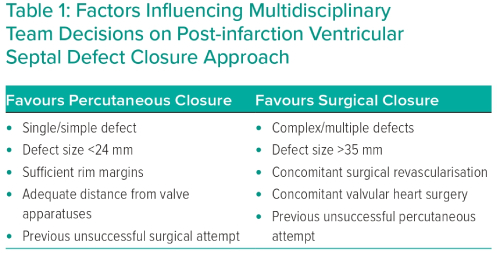
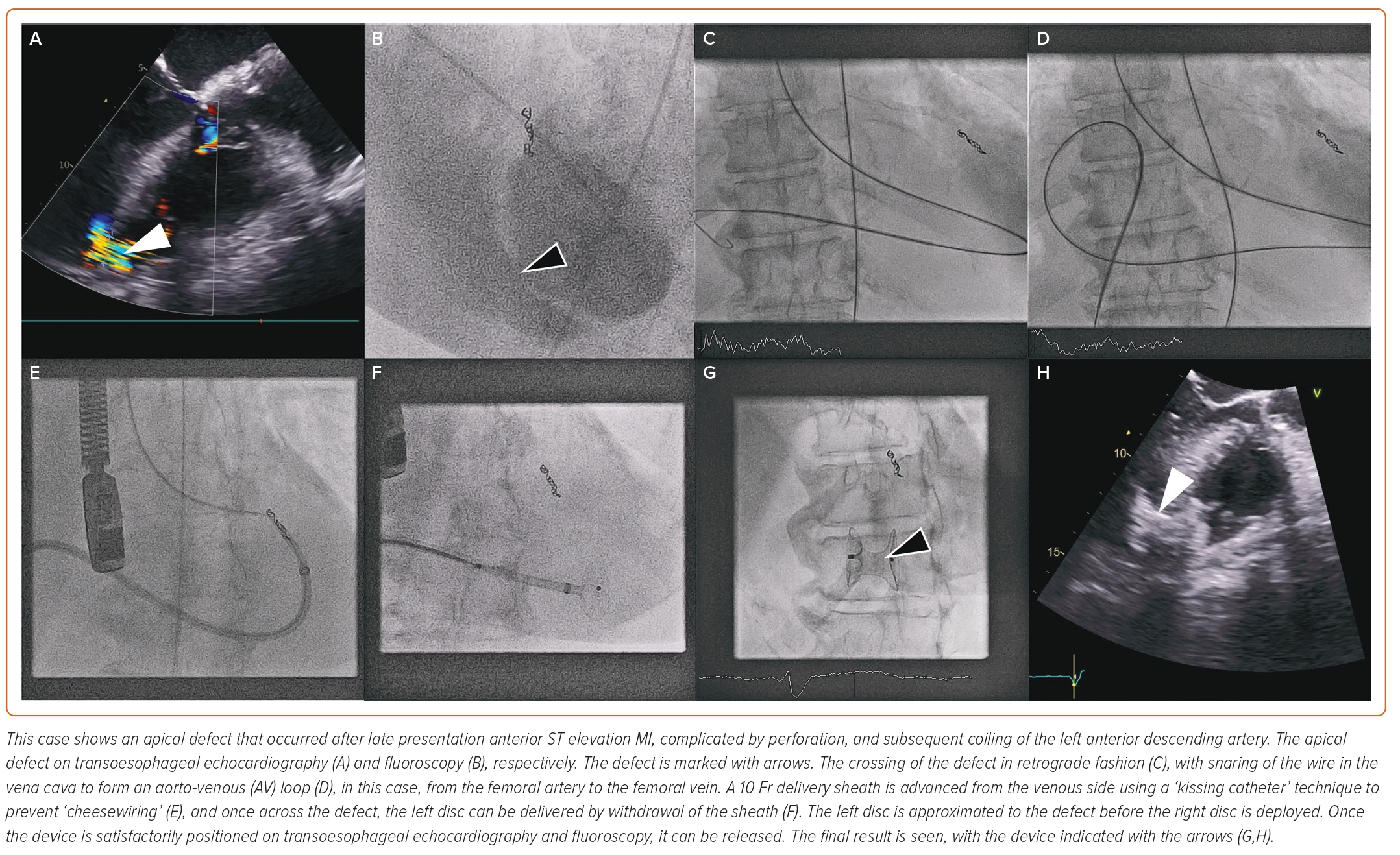
Case selection for percutaneous closure should involve Heart Team discussion with careful analysis of the imaging modalities described above to guide the procedure. Factors that predispose to surgical or percutaneous treatment are shown in Table 1. The procedure is usually performed under general anaesthetic with TOE and fluoroscopic guidance (Figure 2). Two vascular access sites are usually placed, femoral arterial and either femoral or internal jugular vein are most commonly used. A 0.035-inch hydrophilic guidewire is used to cross the defect, directed using a multipurpose or Judkins right 4 diagnostic catheter. Left-to-right crossing is felt to be more easily facilitated given right ventricular trabeculations can make right-to-left wire crossing difficult, particularly if the defect is complex. Once the defect is crossed, the guidewire is advanced away from the tricuspid valve apparatus to the vena cava or pulmonary artery, and then snared to externalise the guidewire and create a stabilising aorto-venous rail. Care must be taken to avoid over-tension on the rail (‘cheesewiring’) further damaging the defect. The internal jugular vein may create a more favourable rail trajectory in posteroseptal or distal apicoseptal defects. Another approach is to create a veno-venous rail from internal jugular to femoral veins, after crossing the PIVSD from the left ventricle via an atrial transeptal puncture.21 The veno-venous approach offers some theoretical advantages (particularly reduced rail torsion and haemodynamic stability related to retrograde crossing of the aortic valve) at the cost of increased procedural complexity. Single-access transarterial approaches may also be considered, using a stiff wire in the pulmonary artery as a rail, and this may offer reduced procedure times with comparable technical success.28 An additional 0.018-inch wire can be placed across the defect in case re-cross is required at any stage.
Device sizing is usually based on TOE guidance at the time of the procedure, factoring in any additional anatomical characteristics obtained from preprocedural imaging. 3D TOE can add further to appropriate device selection, particularly in complex PIVSD. Although sizing balloons may be used, caution is advised, as they risk extending the defect when inflated against necrotic and friable tissue.
Amplatzer occluder devices (Abbott Vascular, Santa Clara, CA, US) are the most commonly reported devices in use. The Amplatzer Post-infarct Muscular VSD Occluder is specifically designed for PIVSD closure, with wider discs and a longer waist than a traditional VSD occluder device. It is advanced via a delivery sheath, more commonly along the venous side of the rail, across the defect. Once appropriately positioned, TOE and contrast fluoroscopy can be used to assess apposition and the degree of shunt reduction before release.
Additional devices can be deployed, if required, to further reduce the shunt, provided there remains adequate tissue rim for capture. Devices can be oversized to enhance capture, pre-empting any further potential defect enlargement post-implant, particularly in cases with extra-septal extension. The potential benefits of oversizing must be weighed against the risk of causing further mechanical disruption with a larger device or impacting adjacent cardiac structures. Complications include arrhythmia, ventricular rupture, device embolisation, device-related haemolysis, bleeding, stroke and death. Device embolisation has been found to occur in 7.6% of percutaneous defect closures, compared with partial patch dehiscence in 13.4% and complete patch dehiscence in 4.3% of surgical closure attempts.5
Future Directions
Sixty-seven years after the first surgical closure of PIVSD, there remain many unanswered questions with regard to optimal management. The rare nature of its occurrence and the inherent severity of its clinical course create challenges in developing good-quality evidence to guide clinicians in treating PIVSD. Retrospective registry data certainly highlight areas in need of review, including preoperative management, timing of closure and technical aspects of each procedure. The heterogeneity of retrospective data often results in more questions than answers being formulated. The formation of an expert steering group and a national/international prospective PIVSD registry may help to address these issues, guide further research and optimise care.
Conclusion
PIVSD is a rare, but life-threatening, complication of AMI that necessitates closure or repair of the defect. Debate remains on the optimal preprocedure optimisation, timing of repair and modality of treatment. These will remain uncertain without prospective evidence to guide clinicians, and in the meantime, Heart Team decision-making remains the best way to decide on treatment for individual patients with the condition. 











Comments