Mechanical circulatory support (MCS) device use for cardiogenic shock (CS) and high-risk percutaneous coronary intervention (HRPCI) has grown over the past decade. However there are ongoing problems, including the standardisation of guidelines for patient selection, protocols for use, weaning and addressing complications.1,2
Among patients undergoing percutaneous coronary intervention (PCI) for acute MI complicated by CS (AMICS), data from a recent cross-sectional study show that 42.7% received an mechanical circulatory support (MCS) device; during the study period of 2015–2017, the use of intravascular microaxial left ventricular assist devices (LVADs) increased substantially while intra-aortic balloon pump (IABP) use decreased significantly.3 In a Premier database analysis, Amin et al. identified that Impella use among percutaneous coronary intervention (PCI) patients requiring MCS rose from approximately 1% in 2008 to 31.9% to 2016, encompassing 9.9% of all PCI procedures performed for MCS.4 The authors did not observe a trend of increasing use of Impella in critically ill patients. This being the case, the overall trend in growing use likely reflects greater utilisation in the context of HRPCI.
Since the introduction of Impella, IABP use has been found to either decline slightly or stay constant. When results from the IABP-SHOCK II trial did not demonstrate a benefit of IABP use on 30-day or 1-year mortality, the use of IABP in cardiogenic shock was downgraded to a class III B recommendation in European guidelines.5 Similarly, in the US, IABP use has been downgraded to a class IIb B recommendation. In addition, a 2005–2014 analysis of the Nationwide Inpatient Sample database found a significant decrease in the use of IABP in patients with acute MI complicated by cardiogenic shock, while the use of both Impella devices and extracorporeal membrane oxygenation (ECMO) increased significantly over this decade.2 The analysis found that patients receiving any MCS were significantly more likely to be younger and to undergo revascularisation; patients receiving MCS also had significantly lower in-hospital mortality than those not receiving MCS.2
Impella Pumps
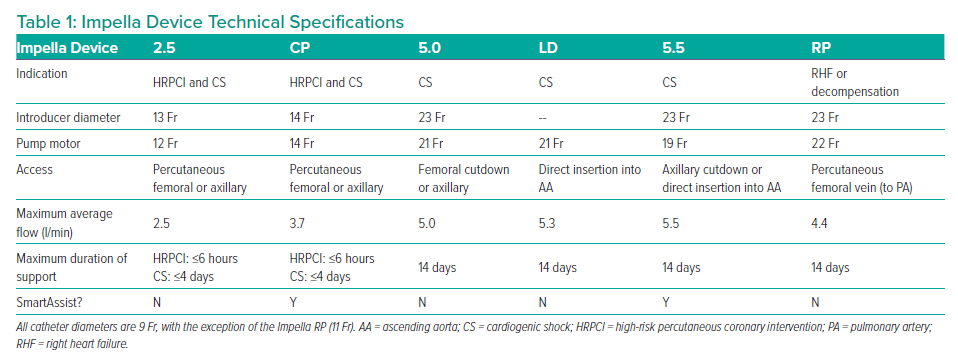
The Impella device (Abiomed) is an intravascular microaxial blood pump that provides temporary MCS to patients, thereby reducing the workload of the heart and improving systemic circulation. Impella devices are used during HRPCI or for treatment of cardiogenic shock following acute MI or cardiac surgery, in the context of cardiomyopathy and in severe myocarditis.6,7
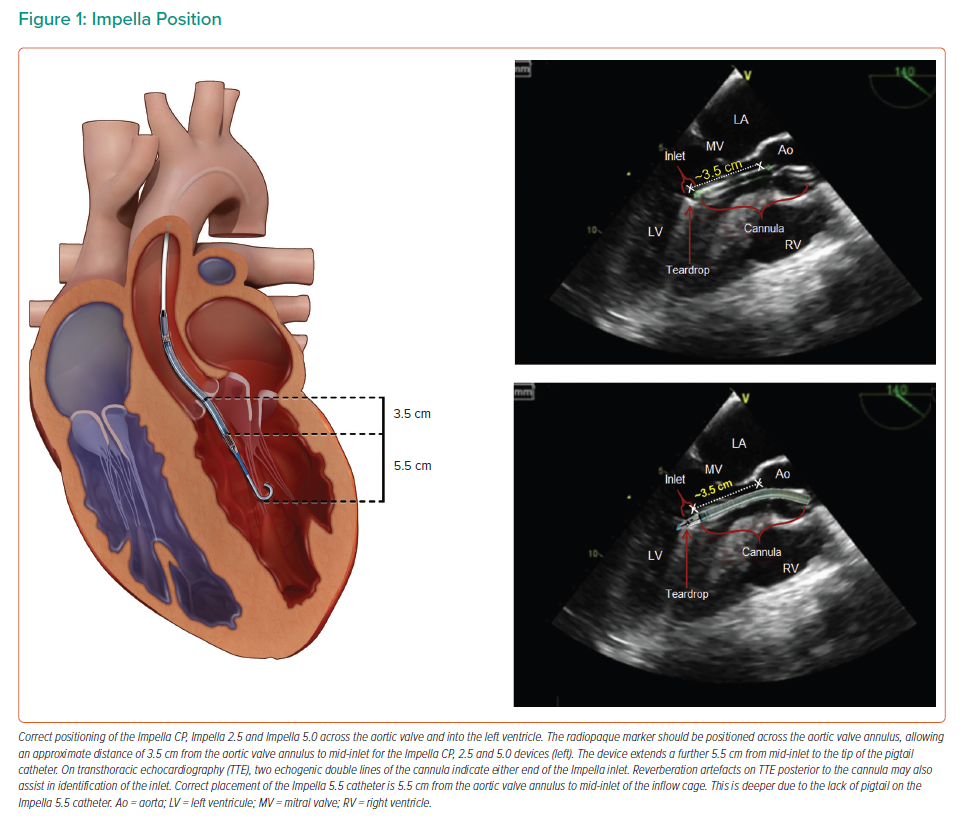
The left-sided Impella devices are microaxial pumps positioned across the aortic valve into the left ventricle (LV) that provide continuous antegrade blood flow from the LV into the ascending aorta, which reduces the workload of the LV and increases cardiac output (Figure 1). The result is improved systemic perfusion and increased coronary flow accompanied by a reduction in myocardial oxygen demand.6,7 A right-sided device delivers blood from the inlet area of the inferior vena cava into the pulmonary artery. The characteristics of each Impella device are detailed in Table 1.
Automated Impella Controller with SmartAssist Technology
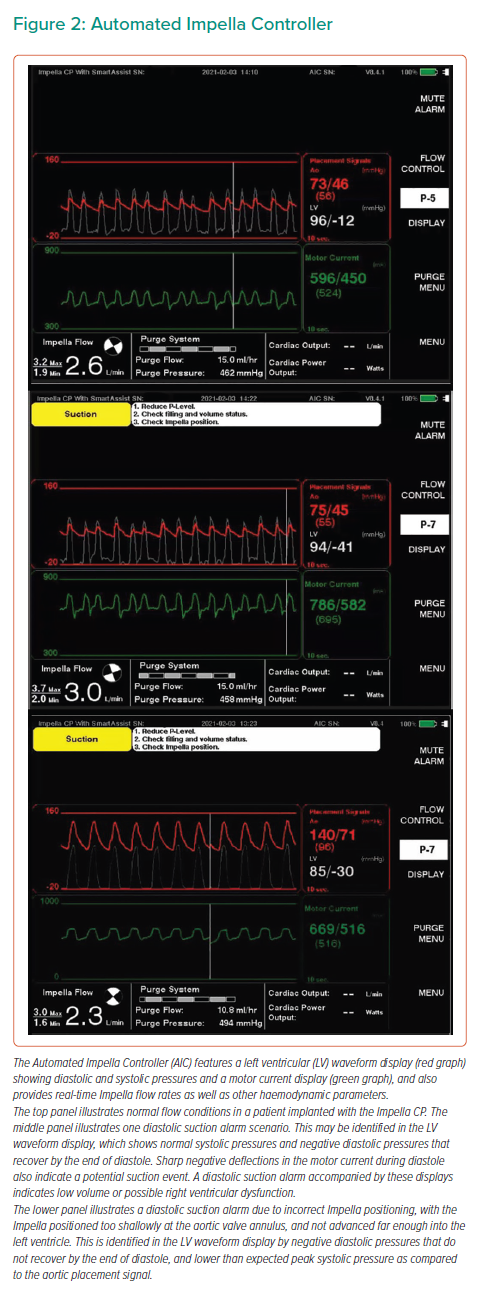
The Automated Impella Controller (AIC) is the primary user control interface for all Impella heart pumps. This technology is accompanied by the Impella Connect, a cloud-based platform that allows for secure, remote viewing and collaborative patient management. The Impella CP and 5.5 are provided with SmartAssist technology, which enables a real-time display of informative pump metrics and device placement on the AIC (Figure 2).
Left-sided Impella devices with SmartAssist technology are equipped with optical sensors that sense the pressure at the outlet of the device (this is the aortic pressure when the devices are in the correct position) and provide the AIC with exact device positioning. In addition, the microaxial motor senses the pressure difference between the inlet and outlet of the Impella device (when placed in the proper position, this reflects the pressure difference between the aorta and left ventricle) to assist in managing and positioning the device. Left-sided SmartAssist technology provides the AIC with data on left ventricular pressure, end-diastolic pressure, continuous cardiac output and cardiac power output (CPO). CPO is a metric that is calculated with the following formula:
(cardiac output × mean arterial pressure) / 451.8 These haemodynamic metrics aid device management and weaning.
The Impella RP with SmartAssist provides the AIC with data on pulmonary artery pressures, central venous pressure and the pulmonary artery pulsatility index (PAPi). PAPi is a metric that is calculated with the following formula: (systolic PAP – diastolic PAP) / right atrial pressure.
High-risk PCI Indication
PCI is considered to be high risk, based on a wide range of criteria involving patient characteristics, including age and comorbidities, lesion characteristics and clinical presentation.9 Patients deemed ineligible for surgery – referred to as surgical turndown patients – are at a higher risk of worse clinical outcomes.10,11 Without the option of surgery, these patients are more likely to undergo PCI. A thorough understanding of patient characteristics and clinical presentation must be considered before any intervention. Predictive risk scores, including SYNTAX and STS scores, have emerged as key tools in the stratification of surgical turndown patients to identify those who could benefit from PCI.12
The PROTECT II study was the first randomised controlled trial (RCT) comparing outcomes with different forms of MCS in patients undergoing HRPCI.13 There was no significant difference in the primary endpoint – 30-day major adverse event rate – in patients treated with the Impella 2.5 or IABP in the PROTECT II trial, which resulted in early trial termination. However, patients supported with the Impella 2.5 showed better performance on a prespecified analysis of the primary endpoint at 90 days, attaining significance in those treated per the protocol, resulting in Food and Drug Administration (FDA) approval for the Impella 2.5 pump in this setting.13 In addition, patients in the Impella arm received more vigorous atherectomy with more runs and longer durations.
A PROTECT II subgroup analysis by Cohen et al. found that patients treated with atherectomy (either trial arm) were older, had significantly higher STS scores and more severe comorbidity burdens, with higher rates of 30-day and 90-day major adverse events, largely driven by higher periprocedural MI rates in patients receiving Impella and undergoing atherectomy.14 Conversely, people receiving an Impella and undergoing atherectomy showed significantly lower rates of repeat revascularisation within 90 days compared to IABP patients.
To date, no robust randomised data exist to support the routine use of Impella pumps for HRPCI. However, the randomised PROTECT IV trial (NCT04763200) is enrolling patients and the CHIP-BCIS3 trial (NCT05003817) will soon be enrolling patients. Both trials are being conducted on non-emergent populations, with patients with cardiogenic shock or acute ST-elevation MI (STEMI) excluded. The PROTECT IV trial is enrolling patients with chronic coronary syndrome or non-ST-elevation MI (NSTEMI) with LVEF ≤40% or acute STEMI ≥24 hours with LVEF ≤30%, with complex PCI planned. CHIP-BCIS3 will enrol patients with a British Cardiovascular Intervention Society Jeopardy Score ≥8 and an LVEF ≤35%, with complex PCI planned. Both trials aim to provide a more definitive answer as to the value of prophylactic Impella support in the HRPCI setting.
Guidelines by major societies regarding the use of MCS during HRPCI are lacking. There have been many previous attempts to objectively risk stratify patients undergoing HRPCI. The algorithm by Kearney et al. is a comprehensive attempt to objectively evaluate these patients.15
Patients at High Bleeding Risk
The decision to use MCS during PCI in patients at a high risk of bleeding must involve a careful assessment that weighs the overall benefits against the risks of thrombotic or ischaemic events, and the treatment plan must be crafted on an individual patient basis.
Benefits include maintaining normal haemodynamics, enabling complete revascularisation, and reducing cardiac mechanical stress. Risks include major bleeding and vascular complications. A comprehensive literature review evaluating the risk of major bleeding and vascular complications in Impella-supported HRPCI revealed a median rate of major bleeding complications of 5.2% and a median rate of major vascular complications of 2.6%.16 Such complications have been associated with a significant increase in mortality and duration of hospital stay.17
Cardiogenic Shock Indication
Mortality rates for patients with CS have historically ranged from 40% to 60% and have not improved despite advancements in therapies.18 Previous trials comparing Impella and IABP in AMICS patients have demonstrated better haemodynamic parameters including cardiac index, cardiac output and mean arterial pressure in Impella patients; however, acute mortality rates were found to be similar between the two devices.19–21 These trials were small and underpowered, highlighting the difficulty of patient recruitment in this context.
In recent years, some institutions have adopted a shock-team approach, in which a designated team follows specific protocols to improve clinical outcomes. A large, quaternary care centre implemented such an approach, utilising early shock team activation, rapid MCS initiation, haemodynamic-guided management and strict protocol adherence.22 Results showed an increase in survival rate from 47% in 2016 to 57.9% in 2017 and 81.3% in 2018.
There is no consensus over the optimal timing for initiating MCS support for patients with cardiogenic shock undergoing PCI. Some evidence suggests that Impella implantation prior to PCI results in improved survival secondary to effective LV unloading and increased systemic and coronary perfusion.23 An analysis of 154 consecutive AMICS patients treated at 38 US hospitals participating in the USpella registry found that early initiation of haemodynamic support before PCI was associated with more complete revascularisation and improved survival.24
The National Cardiogenic Shock Initiative (NCSI) reported a survival to discharge rate of 72%, using a shock protocol that emphasises initiation of Impella support prior to PCI and pulmonary artery catheterisation (PAC) for haemodynamic monitoring.25 NCSI findings also identified the predictive utility of lactate and CPO measurements post procedure to guide clinical decision-making.25,26 PAC has also been recommended for use with MCS in cardiogenic shock to help monitor effectiveness, optimise device settings, determine escalation requirements and assist decision-making on weaning.27
Right Ventricular Failure
The Impella RP is indicated for patients with right heart failure, which can arise secondary to acute MI, myocarditis, acute decompensated heart failure, acute pulmonary embolism and pulmonary hypertension, and following cardiotomy, transplantation and LVAD implantation.
Although invasive, PAC is essential for continuous haemodynamic monitoring of pulmonary artery pressure and other metrics to ensure that adequate support is being administered to the patient.28
The Impella RP can be used in tandem with a left-sided Impella device. The use of two Impella devices concurrently has demonstrated decreased LV filling pressures and improved cardiac output for cardiogenic shock patients, although reported data on this use is limited and future studies are required.29
Quantifying right ventricular (RV) failure and identifying patients in need of an RV assist device is not always easy. Many noninvasive and invasive parameters have been used. One such invasive haemodynamic parameter is PAPi, which was first used in patients with RV failure after inferior MI, but has since been shown to be helpful in many scenarios including nonischaemic cardiogenic shock.30–32 Various PAPi cut-off values have been used to classify patients in need of RV support; the most commonly used cut-off value of PAPi ≤0.9 has been shown to have 100% sensitivity and 98% specificity for predicting in-hospital mortality and/or requirement for RV support.30
Impella Implantation Technique
Femoral Access
The Impella 2.5 and Impella CP are inserted using 13 Fr and 14 Fr sheaths, respectively, via a retrograde femoral arterial approach. The catheter is inserted percutaneously through the femoral artery into the ascending aorta, across the aortic valve and into the left ventricle, guided by fluoroscopic or echocardiographic imaging. The femoral approach is the most common insertion route for Impella 2.5 and CP, but is not indicated for patients with severe peripheral arterial disease (PAD) or for those requiring longer-term support that necessitates lengthy immobilisation.33 The Impella 5.0, which requires a 23 Fr sheath, may be inserted via surgical cutdown of the femoral artery but is more commonly inserted via axillary cutdown.
Iliofemoral angiographic evaluation is warranted before any consideration of large-bore femoral access. For HRPCI patients, this evaluation should be performed during the index diagnostic coronary angiogram procedure. This allows for a discussion of MCS placement technique as part of the revascularisation strategy. The evaluation could be performed during the staged HRPCI procedure, but it is preferable to perform this before the PCI procedure to avoid surprises and to adequately assess the risks of PCI. In our experience, the best outcomes are achieved with thorough pre-procedure planning.
Axillary Access
When Impella 2.5 or Impella CP femoral access is precluded due to PAD, it may be feasible to use the axillary artery as an alternative access site.34,35 Axillary insertion is not as restrictive to ambulation as femoral artery insertion – an important factor for patients requiring longer-term support who would otherwise be immobilised. Impella 2.5 and CP can be placed by percutaneous peripheral insertion into the axillary artery, while the large sheaths of Impella 5.0 and Impella 5.5 necessitate surgical cutdown of the axillary artery.
Surgical and Transcaval Implantation
Because of the 23 Fr sheath required to insert Impella 5.0, 5.5 and LD, these devices must be placed by a surgical cutdown arteriotomy or via a transcaval approach by highly skilled operators with transcaval access experience. The Impella LD is exclusively surgically inserted into the ascending aorta through a 10 mm vascular graft and advanced across the valve into the left ventricle.33 The Impella 5.0 and 5.5 may be implanted via a transcaval approach for patients with prohibitive iliac artery anatomy or disease, or through axillary cutdown or direct insertion into the ascending aorta through either the supraclavicular fossa or the midclavicular intercostal space.36
Right Heart Access
The Impella RP is a 23 Fr pump on an 11 Fr catheter that is inserted percutaneously through the femoral vein into the pulmonary artery, guided by fluoroscopic imaging. The inflow portion of the catheter is placed in the inferior vena cava, and the nitinol cannula is advanced across the right atrium, tricuspid valve and pulmonary valve to position the outflow portion of the catheter in the main pulmonary artery.37
Left Ventricular Device Repositioning
Correct initial device placement can be confirmed by imaging with fluoroscopy, although bedside echocardiography after the patient has been moved from the cath lab should be mandatory. If repositioning is required, transthoracic echocardiography (TTE) can be used to visualise the device. Repositioning without visualisation, although generally not recommended except in emergencies, can be accomplished by retracting the cannula until the diastolic pressure normalises, or through using SmartAssist technology on the Impella CP or Impella 5.5 devices.38 If the TTE images are difficult to interpret, repositioning based on fluoroscopy or transoesophageal echocardiography may also be considered.
Single Access Technique
Jason Wollmuth first proposed the Single-access for Hi-risk PCI (SHiP) technique, where a PCI catheter up to 7 Fr is inserted through the valve of the 14 Fr Impella CP sheath parallel to the catheter.39 In a case series of 17 patients undergoing SHiP, Wollmuth et al. reported no bleeding events during the procedure or after the removal of the sheath, although in one patient iliac thrombus was noted with Impella catheter removal several days after PCI.39
Device Weaning and Escalation
As there are no universally accepted guidelines for weaning, strategies vary between individual patients and rely on haemodynamic parameters predictive of patient outcomes. The majority of patients receiving Impella support during HRPCI do not require an extended duration of support and undergo device removal at the end of the procedure.
CPO has been shown to be the strongest predictor of mortality in patients receiving MCS for cardiogenic shock, and can be used to guide weaning.25
At our facility, the interventional cardiologist supervises the weaning process over the course of a few hours. Box 1 describes our weaning and escalation process for Impella support for cardiogenic shock.
It should be noted that prolonged high or low levels of Impella support can be harmful. Low support levels for long periods of time can precipitate thrombosis. Higher levels of support can put the patient at risk of haemolytic events. The true incidence of haemolysis is not well reported and is likely to vary depending on factors such as Impella positioning and patient volume status in addition to the level of support.40
Box 1. Impella Weaning/Escalation Protocol at Ascension St John Hospital and Medical Center
- Consider weaning from Impella support if cardiac power output >0.6 W and other haemodynamic parameters are adequate without the use of vasopressors and high-dose inotropes.
- Reduce Impella performance level (P-level) every hour.
- Measure mixed venous oxygen saturation (SvO2), lactic acid and urine output every hour to ensure that the patient continues to have adequate cardiac output.
- With every change in P-level, calculate cardiac power output, pulmonary artery pulsatility index (PAPi), and systemic vascular resistance (SVR).
- Continue weaning if the SvO2 remains >50%, cardiac power output is >0.6 watts, mean arterial pressure is >60 mmHg and lactic acid remains <3 mmol/l. If these values are not achieved, initiate positive inotropic therapies to expedite the weaning of the Impella device.
- If the patient develops tachycardia, arrhythmia-related decreases in mean arterial pressure, decreased urine output, increasing lactic acidosis or worsening pulmonary artery pressures, consider increasing Impella support as necessary to stabilise the patient before reattempting weaning.
- If CPO <0.6 W, consider escalation from Impella 2.5 or CP to Impella 5.0 or 5.5 depending on the patient’s characteristics. Support may be continued until native heart recovery or as a bridge to durable therapy, such as a long-term LVAD or transplantation (in the absence of multi-organ failure).
Patients with Impella support devices should be monitored closely for haemolysis. It is routine at our institution to check haemolysis lab findings (i.e. plasma-free haemoglobin, lactate dehydrogenase, haptoglobin and bilirubin) every 6–12 hours, with plasma-free haemoglobin identified as being highly sensitive and specific for haemolysis in patients treated with Impella in line with a recent analysis by Esposito et al.41 The frequency should be adjusted as necessary.
Patients with significant haemolysis are at risk of kidney injury so early detection can be organ saving. These patients should be monitored more frequently whereas those without signs of significant haemolysis can have lab tests carried out less often.
Haemolysis can be treated with confirmation of correct device positioning, administration of IV fluids and reduction in the level of Impella support; however, in many patients, expedited explantation is necessary. Initiation or titration of inotropes is necessary in many of these patients for successful reduction in support and device removal.42
Closure Strategies
Vascular closure devices (VCDs) have arisen as a potential tool for reducing complications associated with large-bore femoral artery closure, including uncontrolled bleeding, patient immobilisation, increased length of hospital stay and vascular complications.
Of note is the MANTA VCD (Teleflex), a collagen-based VCD designed for large-bore femoral access closure, which received FDA approval in 2019. Outcomes with the MANTA and the double-pre-close closure technique were compared in patients undergoing transcatheter aortic valve replacement (TAVR) in the MASH RCT. Similar performance on the primary endpoint of access-related vascular complications was reported; however, those receiving the MANTA had a significantly shorter time to haemostasis and significantly lower rate of modified VCD failure.43
Many clinicians find it useful to ‘pre-close’ large-bore access sites using two sequentially placed Perclose ProGlide devices (Abbott Vascular Devices) at the 10 o’clock and 2 o’clock positions before placing the Impella sheath. This pre-close technique has been found to be useful in achieving haemostasis in immediate as well as delayed closure cases.44
In patients at a high risk of bleeding, the dry closure technique with balloon tamponade is recommended to prevent excessive bleeding. This method involves the advancement and subsequent inflation of a balloon proximal to the access site, followed by slow balloon deflation until haemostasis is achieved. Perclose sutures are then used to close the access site.45
Anticoagulation Regimens
Systemic anticoagulation is required with Impella use to decrease the risk of thrombus formation along the length of the catheter or on the body of the Impella device. A purge solution containing heparin flows through the Impella catheter in the opposite direction of the patient’s blood to generate a pressure barrier that prevents blood from entering the motor, and to keep purge gap regions clear of debris. Strategies for IV-based anticoagulation must take into account purge flow rates with the Impella, pre-existing coagulopathy and heparin allergies.
The viscosity and flow rate of the purge solution is determined by its dextrose concentration. When the concentration of dextrose is low, the purge flow rate will be faster due to the lower viscosity, resulting in greater delivery of heparin to the patient. Higher dextrose concentrations yield greater viscosity and a slower purge flow rate that delivers less heparin to the patient.
The current recommendation for the initial purge solution is a heparin concentration of 25 U/ml in 5% dextrose. In some patients, the addition of titratable, supplemental IV heparin is required to provide optimal anticoagulation.
In patients with heparin-induced thrombocytopenia, an anticoagulant-free purge solution is recommended with an alternative systemic anticoagulant. In a recent case series at the Cleveland Clinic, nine patients with suspected or proven heparin-induced thrombocytopenia received Impella CP support with low-concentration bivalirudin added to the purge in addition to systemic bivalirudin.46
Complications
Femoral insertion of the Impella 2.5 or Impella CP involves standard catheterisation procedures except for the requirement of a large-bore 13 or 14 Fr sheath, which can result in haematoma formation, uncontrolled bleeding and injury to the vasculature that may necessitate surgery.
Haemostatic complications can arise from all forms of MCS because they involve the placement of a foreign object and shear forces on blood flowing through the device. Platelet aggregation, thrombosis, mechanical haemolysis and thrombocytopenia due to heparin use are potential complications that must be managed for each patient.
A comprehensive literature review of Impella use during HRPCI demonstrated that, over time, rates of major bleeding complications have varied considerably, while transfusion rates have decreased and vascular complication rates have remained consistently below 5%.16
Patient selection in view of bleeding risk as well as careful access vessel selection and approach are important to mitigate the risk of access-related bleeding and vascular complications. Vascular access techniques that include the use of fluoroscopy, ultrasound, micropuncture, angiography and vascular closure devices help optimise patient outcomes.47
Maintaining femoral skills (as radial access for PCI has come to predominate) is paramount. Novel emerging technologies and techniques such as the MANTA VCD and the single-access technique may have potential utility in alleviating the risks of large bore access.
Haemolysis can occur with Impella devices so avoiding suction alarms, daily imaging and maintaining adequate fluid status are imperative to reduce its likelihood. Pulmonary artery diastolic pressures should be maintained between 15 mmHg and 20 mmHg to ensure adequate intravascular volume. Haemolysis is monitored via daily laboratory values including but not limited to LDH, plasma free haemoglobin and haptoglobin. All patients should be fitted with a Foley catheter to monitor urine output as a marker of adequate perfusion as well as haemolysis. It is important to note that daily echocardiograms should be done to ensure appropriate positioning of the Impella device; haemolysis may be an early indicator of poor Impella placement which may lead to decreased cardiac output and poor outcomes.
Future Directions
In December 2020, the FDA granted 510(k) clearance to the Impella XR Sheath, a low-profile sheath made of nitinol braids designed to be inserted at 10 Fr and to expand and recoil for simplified percutaneous insertion with the Impella 2.5.48 The reduction in size is intended to lower the incidence of vascular and bleeding complications related to large-bore access.
The Impella ECP heart pump, which is inserted and removed through a 9Fr sheath, has completed the first stage of its early feasibility study and was granted breakthrough device designation from the FDA in August 2021. The Impella ECP expands after insertion to provide peak flows greater than 3.5 l/min.
For patients in cardiorespiratory failure, the main percutaneous extracorporeal life support system in use is venoarterial ECMO. The ECMO circuit provides temporary MCS along with gas exchange; however, veno-arterial-ECMO circuits result in increased afterload, which is not sustainable for patients with LV contractile dysfunction. Abiomed’s Breethe OXY-1 System (an all-in-one cardiopulmonary bypass system that can be used in conjunction with the Impella, and allows patient ambulation) received 510(k) clearance in October 2020.49 The combination of ECMO with Impella, a therapy known as ECpella, provides venting for the left ventricle in order to continue forward flow from the left ventricle.50,51 Further investigation is warranted to elucidate the risk-benefit profile of the ECpella approach.
Upcoming Trials
Upcoming RCTs will provide much-needed evidence assessing outcomes with Impella use in PCI.
STEMI DTU is a prospective, multicentre, two-arm trial expected to enrol 668 patients undergoing treatment for STEMI and not in cardiogenic shock. Patients will be randomised 1:1 to either 30 minutes of unloading with Impella CP prior to reperfusion, or the standard care, which is immediate reperfusion. The pivotal trial follows the DTU STEMI pilot study, which showed no prohibitive safety signals to the unloading-before-reperfusion approach.52
The upcoming PROTECT IV RCT will randomise patients with LVEF ≤40% and chronic coronary syndrome or NSTEMI or LVEF ≤30% and STEMI >24 hours, who were selected for complex PCI after heart team discussion to HRPCI with Impella or standard care. The first patient was enrolled, at our institution, in April 2021. The trial aims to validate the best practices learned for Impella-supported HRPCI since the completion of the PROTECT II trial more than a decade ago.
Conclusion
The use of the Impella devices for cardiogenic shock and HRPCI has increased since their inception. The ideal access site, including implantation and closure techniques, requires endovascular expertise and nursing experience is needed to ensure successful patient outcomes. Patients on pump require constant haemodynamic and haematological monitoring to ensure adequate response to therapy and early detection in bleeding complications. Furthermore, new research is on the horizon to help us better understand how these devices can advance patient care.