In acute heart failure, unloading the left ventricle (LV) by percutaneous LV assist devices (pLVAD) reduces LV filling pressures and volumes, as well as cardiac workload and wall stress, which, in turn, reduces myocardial oxygen demand and increases coronary perfusion. Dr Mavropoulos shared previous mechanistic studies that examined the effects of unloading at the intracellular level, including changes in the expression of calcium-handling proteins, Ca2+-ATPase activity and mitochondrial dynamics.1,2
Dr Mavropoulos described the cardiac extracellular matrix (ECM) as a dynamic scaffold of macromolecules surrounding the cells that can transmit the forces that act upon the heart and signals to the cardiomyocytes in response to these forces. The ECM acts as a modulator of cell signalling, adhesion, angiogenesis and fibrosis.
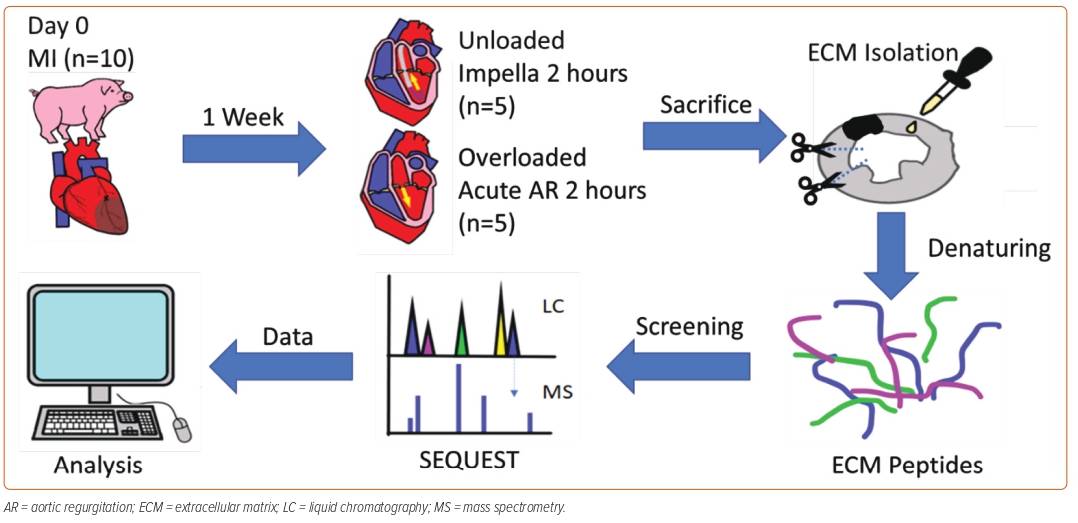
In his current study, Dr Mavropoulos hypothesised that the change in the composition of LV ECM proteins is another mechanism underlying the benefits provided to the heart by acute LV unloading using a pLVAD. MI was induced in 10 Yorkshire pigs using an inflated balloon catheter in the mid-left anterior descending artery for 90 min. After 7 days, the pigs were split into two groups: one group was unloaded with Impella to create an unloaded state of the LV, and the other underwent mechanical induction of acute aortic regurgitation to create an overloaded state. After 2 hours, all pigs were killed and their cardiac tissues harvested for ECM protein extraction. The resulting denatured peptides were screened using liquid chromatography–mass spectrometry (LC-MS) and subjected to computational analysis (Figure 1).
The LC-MS analysis showed that of 986 proteins identified in the ECM isolates, using cut-off values of a ≥50% increase or decrease in expression levels (p=0.05), 39 ECM proteins were identified as differentially expressed between the unloaded and overloaded hearts. For the 39 proteins identified, gene ontology analysis by molecular function showed there was increased protein expression of RNA- and lipid-binding proteins in the unloaded group. Gene ontology analysis by biological processes showed a relative increase in the expression of proteins involved in cell differentiation, vesicle transport and programmed cell death processes in the unloaded group.
Dr Mavropoulos concluded that there were significant differences in the protein composition of the cardiac ECM between acutely overloaded and unloaded myocardium. The differentially expressed proteins are those largely involved with the molecular functions of RNA and lipid binding and the biological processes of cell differentiation, vesicle transport and programmed cell death. The study findings suggest that the possible initiation of changes in the cardiac cell phenotype is in response to the unloading process and that the benefits induced by pLVAD may persist beyond device removal.
Dr Mavropoulos concluded that this study calls for further characterisation of the effects of the differentially expressed proteins on the cardiac tissue, which could lead to identification of potential therapeutic targets, including the possible use of Impella for diagnostic and discovery purposes.