Dr Swain is the recipient of the Young Investigator Award awarded by the A-CURE Faculty for the scientific merit and innovation of her research.
A previous study showed that mechanical preconditioning with left ventricular (LV) unloading with a mechanical circulatory support device reduces infarct size and wall stress in acute preclinical models of MI compared with primary reperfusion, despite a delay in reperfusion by 60 min.1 Another recent research study found that transvalvular unloading and delayed reperfusion preserves myocardial energy substrate utilisation and protects mitochondrial structure and function in acute MI.2
Dr Swain outlined the molecular changes during ischaemia versus after reperfusion: ischaemia triggers metabolic changes within the cells and a switch to anaerobic glycolysis.3 This, in turn, increases glucose uptake, with an associated rise in lactate that lowers cellular pH, closing the mitochondrial permeability transition pores (MPTPs).3 Reperfusion washes out lactate and opens the MPTPs, increasing the loss of calcium and leading to cell hypoxia and apoptosis. Cellular signalling of oxygen is regulated by hypoxia-inducible factor (HIF)-1α.4
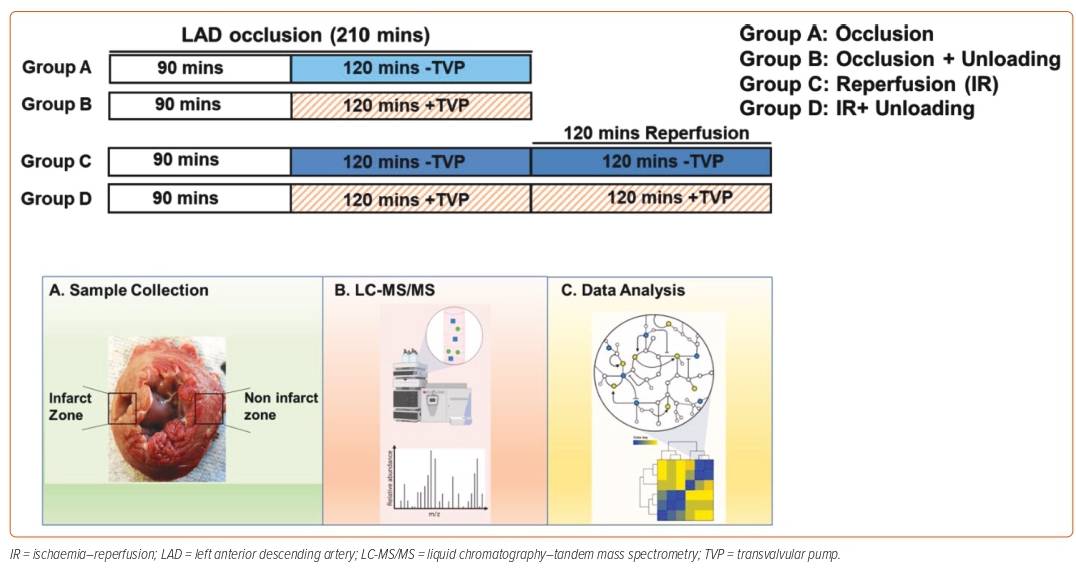
The hypothesis of Dr Swain’s current study was that LV unloading reduces infarct size and promotes myocardial recovery by first reducing ischaemic injury and then further limiting reperfusion injury. Her study used the pig model of acute MI, with pigs divided into four groups. Group A underwent induction of a total of 210 minutes of left anterior descending coronary artery (LAD) occlusion without reperfusion; Group B underwent a total of 210 minutes of LAD occlusion, but with added LV unloading by Impella CP in the last 120 minutes of ischemia; Group C underwent a total of 210 minutes of LAD occlusion followed by 120 minutes of reperfusion; and Group D underwent 210 minutes of LAD occlusion with the last 120 minutes of LV unloading, and were then re-perfused for 120 minutes with continued LV unloading with Impella CP. At the end of the study, the myocardial infarct size of all pigs was quantified and tissue samples were collected. Tissues samples underwent liquid chromatography–mass spectrometry to identify the differential metabolites (Figure 1).
Dr Swain presented the results of her study, which showed that hearts with LV unloading during ischemia (Groups B and D) had smaller infarct size whether or not there was reperfusion. The majority of myocardial injury occurs after reperfusion; however, by unloading the myocardium with a transvalvular device, the infarct size after reperfusion is significantly reduced.
Based on the metabolomics data, concentrations of lactate and glucose, as markers of anaerobic glycolysis, were significantly lower in the hearts that had LV unloading during ischemia (Groups B and D), suggesting LV unloading reduces anaerobic glycolysis before reperfusion, meaning reduced ischaemic injury. Further, LV unloading during ischemia reduced energy demand, as indicated by lower concentrations of ATP and ADP, as well as their precursors, indicating reduced workload and metabolic demand.
Levels of HIF-1α, a molecular marker of ischaemia, were measured in tissue samples. During ischaemia, HIF-1α levels were decreased in the LV unloading group (Group B versus Group A), but were unchanged in the reperfused groups (Group D versus Group C). Dr Swain’s findings suggest that LV unloading limits myocardial HIF-1α expression during ischemia, but stabilises HIF-1α levels after reperfusion. Analysis of other targets of HIF-1α associated with glycolysis showed that LV unloading reduces anaerobic glycolysis before reperfusion.
Dr Swain concluded that her findings show that LV unloading for prolonged periods of time is likely to be well tolerated and safe by providing systemic haemodynamic support while normalising myocardium metabolism and thereby making myocardium recovery more likely.