Approximately 1.8 % of the US population has mitral valve disease,1 and an estimated 106,000 surgeries per year are for the treatment of valvular heart disease.2 A total of 210,529 mitral surgeries were performed from 2000 to 2007, averaging approximately 30,000 mitral valve surgeries yearly in the US, accounting for both combined and isolated mitral procedures, with approximately 60 % of surgeries using a bioprosthetic valve.3 Unfortunately, the durability of surgical mitral valve repairs and replacement are limited. There is a 25 % rate of significant mitral regurgitation recurrence in surgical repairs at 2 years and 44 % rate of primary valvular failure at 15 years,4 leaving the patient with either symptomatic mitral regurgitation, stenosis or both.5
Elderly patients with significant prosthetic mitral valve disease are often non-ideal candidates for cardiac surgery as advanced age, multiple comorbidities and prior sternotomies can elevate the risk of mitral valve replacement to 7.4–15.1 % mortality.6–8 In fact, in a multivariable analysis, independent predictors of in-hospital mortality, including New York Heart Association III and IV symptoms (OR 3.19; p=0.012) as well as more than re-operation (OR 2.59; p=0.058).7 In another retrospective analysis of elderly patients with mitral valve surgery, previous mitral valve replacement increased the 30-day mortality rate nine-fold (p=0.013). Moreover, 30-day mortality was compounded by chronic renal failure (OR 8.041; p=0.022), peripheral vascular disease (OR 5.976; p=0.025) and increasing age (OR 1.077; p=0.013), all of which frequently complicate the clinical course of elderly patients.6
Degenerative changes in surgically repaired mitral valves or valve replacements can result in severe regurgitation or stenosis-causing symptomatic heart failure. Degeneration of such surgical repairs may present with up to 25 % moderate–severe mitral regurgitation within 3–4 years post-repair and most bioprosthetic mitral prostheses sustain 10–15 years usage before degenerating.5,9,10 Furthermore, repeat cardiac surgery can be high-risk due to advanced age and the accumulated comorbidities associated with the elderly.11 Evolving transcatheter heart technology has expanded the options of treating degenerated post-surgical valves to using transcatheter heart valve (THV) implantation for the mitral position.12–26
State-of-the-art transcatheter therapy in failed mitral valve surgeries use balloon-expandable THVs, mostly from the Edwards Lifesciences’ family of valves (SAPIEN, SAPIEN XT, SAPIEN 3). In general, the first step in the implantation process is identifying the correct prosthesis size for the intended location of implantation. For patients with failed surgical valves, identifying the manufacturer and model of valve are essential. Once the internal dimensions have been identified, a valve may be chosen and the options are readily available in the form of a mobile app.27 Alternatively, a gated CT scan can be used to approximate the internal dimensions and a valve can be selected appropriately.
While the selection of new prosthesis for degenerated surgical valves is relatively simple, selection of valves for degenerated repairs and mitral rings is more nuanced. Some rings are complete and some are incomplete and there is not a consensus as to whether or not to size a ring to the broadest dimension, the commissure-to-commissure distance, or to size according to the area within the ring similar to the way aortic valves are sized to an annulus. In general, we suggest sizing the valve to circumscribed area within the confines of the ring with the understanding that the ring is usually oval or ‘D’ shaped. The prosthesis usually conforms to the ring but must fill out the commissure-to-commissure distance to avoid a perivalvular leak.
If there is concern that the commissure-to-commissure distance is very broad (e.g. area of 390 mm but a commissure-to-commissure distance of 30 mm), we would choose a larger valve (26 mm Sapien 3). Sizing of mitral rings continues to be a work in progress in attempts to optimise effective orifice area and minimise perivalvular leak. Of note, the last challenge is when the mitral annular ring is too large for the off-label use of an Edwards SAPIEN 3. To date, we are aware of only the Tiara™ valve (Neovasc) being used for the treatment of valve-in-ring degenerated repairs.
In addition to determining the size of prosthesis, the possibility of left ventricular outflow tract (LVOT) obstruction must be thoroughly evaluated using CT, and, as of yet, protocols using advanced 3D modelling tools are still in development for evaluating LVOT obstruction.28 As this remains a significant vulnerability in transcatheter mitral valve therapies, clinicians have been prompted to develop techniques to avoid LVOT obstruction, either by alcohol septal ablation or intentional percutaneous laceration of the anterior mitral leaflet.29,30
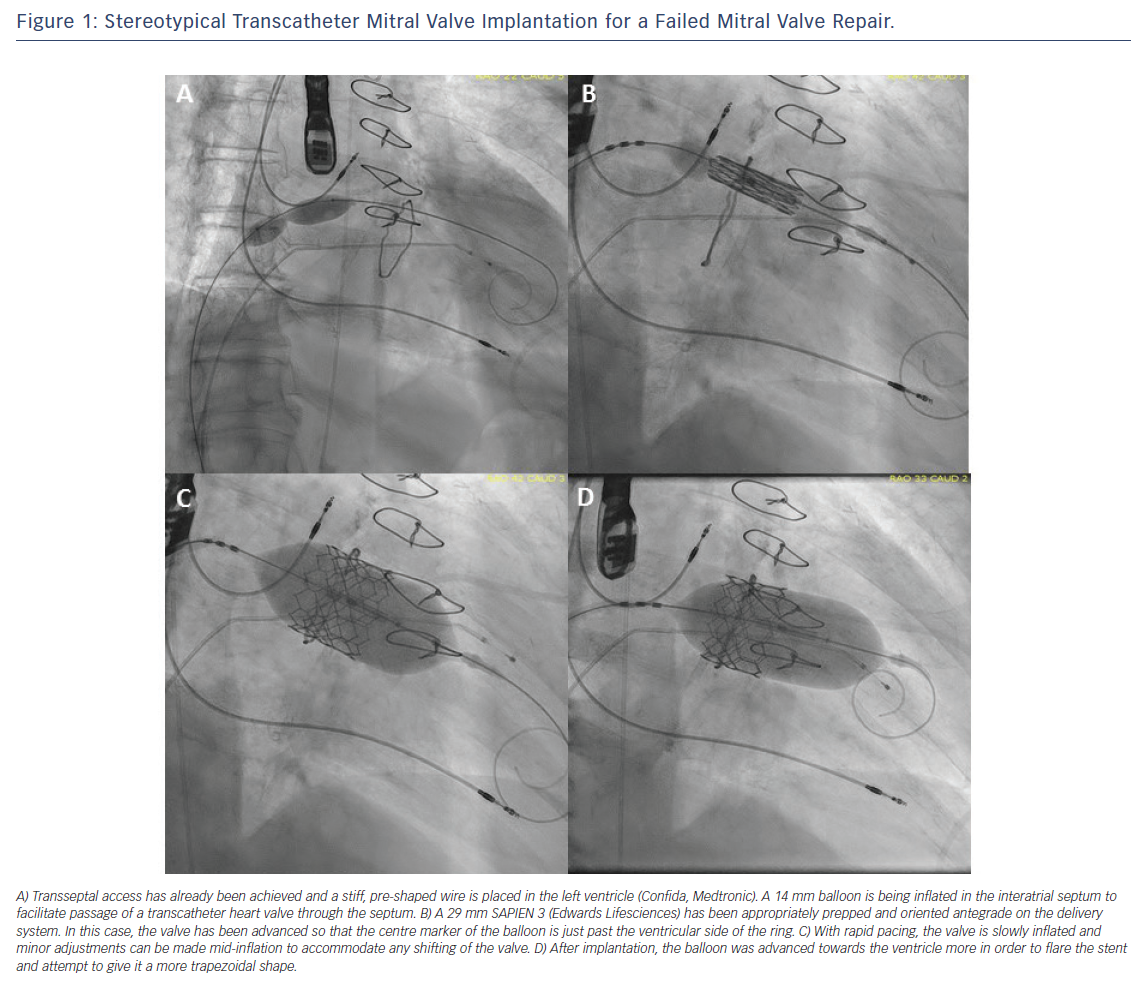
For the procedural technique, transapical access uses a standard thoracotomy similar to what is used for transapical transcatheter aortic valve replacement access. Once the apex of the heart is exposed, pledgeted sutures are placed and an 18 gauge needle is used to access the apex. Once a wire is passed from the left ventricular (LV) apex to the left atrium, a balloon-tipped catheter can be used to ensure that no chordal entrapment occurred with passing the wire. Next, exchange for a large, dedicated delivery sheath is performed over a stiff wire and the valve is prepped and deployed. Transapical access has the advantage of providing co-axial delivery of a prosthesis in the mitral space and makes deployment simple. The balloon inflation is still performed with a run of rapid pacing similar to the implantation of a balloon-expandable valve.
For transseptal delivery of a balloon-expandable valve prosthesis, transoesophageal imaging plays a larger role. Transseptal access with a mid-posterior bias is helpful in establishing a favourable trajectory for the valve. Subsequently, balloon septostomy is performed using a 12–14 mm peripheral balloon to enable smooth crossing of the valve. The majority of cases are performed with transseptal access only because of the narrow delivery profile of the SAPIEN 3 delivery system. In the past, small sheaths have been placed at the ventricular apex to facilitate transseptal crossing by externalisation of the wire through the apex and providing countertraction to the delivery catheter. After the delivery sheath is in place, the valve is mounted on the balloon catheter in an orientation that allows blood to flow from the left atrium to the LV. After the valve is mounted on the balloon and crosses the atrial septum, it is deployed with a slow gradual inflation and simultaneous rapid pacing (Figure 1).
The largest published cohort of mitral valve-in-valve is comprised of 33 patients with median follow up of 723 days.12 Patients had a mean age of 81 ± 6 years and a mean Society of Thoracic Surgeons score of 12.2 ± 6.9 %. The mode of THV delivery was transapical in all patients, with most patients being extubated in the operating room (87 %). Valve implantation resulted in a significant decrease of mitral regurgitation and the mean gradient decreased from a mean of 11.1 mmHg to 6.9 mmHg post-procedure. Mortality was found to be 9.6 % and there was a 26.1 % rate of Valve Academic Research Consortium (VARC)-2 defined major bleeding observed in the cohort. Subsequent analysis from the same institution with median follow up of 4.4 years showed a survival of <40 %, primarily attributed to the elderly nature of the population and the multiple associated comorbidities.31 Notably, this series found a 6.5 % rate of valve thrombosis.
Other transcatheter valve-in-valve series have been described using a transseptal approach, either with an apical rail or without.32–34 One series describes the procedural success of nine patients, using the same technique as described in this manuscript, with an apical access used as an external rail to facilitate Melody™ valve (Medtronic) implantation. In their series, two of the nine patients required thoracotomy for haemothorax and their occluder device of choice was the Amplatzer™ vascular plug (St. Jude Medical).
Two other series, a 17- and 4-patient clinical series demonstrated the feasibility of performing transcatheter mitral valve replacement (TMVR) without the apical access and via transseptal access alone. In the 17-patient series, they describe an 82 % rate of procedural success with one procedural death for a patient in cardiogenic shock, a valve migration and one patient with moderate paravalvular leak.34 Ultimately, it appears that TMVR for failed surgical prostheses or surgical repairs is technically feasible and achieves acceptable immediate results.
The latest series of transcatheter valve implantation describes the outcomes of 48 patients in a single-centre experience that encompasses implantation in not only degenerated mitral bioprostheses and annuloplasty repairs but also mitral stenosis due to annular calcification.35 In this cohort, the authors emphasise a procedural modification where use of pre-shaped nitinol wires improved the safety of the mitral valve-in-valve/ring delivery. With new variation, they report 100 % success (19/19 cases), shorter procedure time (pre-modification 114 ± 28 minutes versus post-modification 86 ± 30 minutes; p<0.05) and numerically lower numbers of major bleeding, ventricular perforation and death. The mean gradient at 30-day follow up was 7.0 mmHg. This single-centre series illustrates that the early experience does include some major complications including ventricular perforation and valve embolisation, however, for patients without surgical options the mortality remains relatively low at 30 days (Table 1).
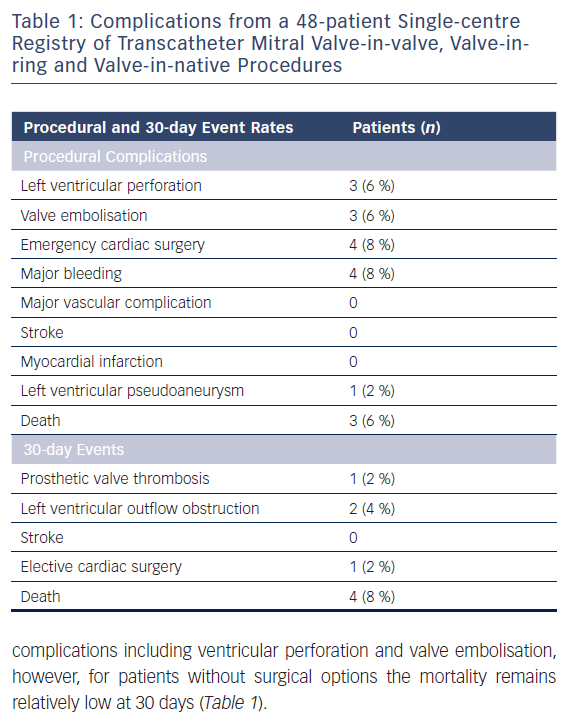
The 30-day mortality rate for redo-mitral valve replacement was 10.1 % and the mortality for the percutaneous cohort is 8 %, illustrating the burden of comorbidities of the patient population.6,35 While this single-centre series encompasses most of the major complications, the prospect of late migration with subsequent embolisation remains a concern.36 To address this issue, operators have advocated implanting the THV on the ventricular side of the prosthesis or ring and flare this side to prevent migration.
Conclusion
Transcatheter mitral valve implantation for post-surgical failures is a feasible alternative to repeat surgery. Transapical and transseptal delivery routes are both feasible, with the latter associated with a relatively low rate of morbidity. Key elements to success include rigorous anatomic evaluation for prosthesis size and possibility of LVOT obstruction. We are currently seeing a number of dedicated transcatheter mitral valves for treating native mitral pathology delivered transapically, and transseptal delivery may possibly grow.







