Considering the unacceptably high mortality rate of patients with cardiogenic shock (CS) and the absence of widespread improvements in survival over recent decades, the time has arrived for the cardiovascular community to embrace a ‘combat’ approach to CS.1 In the past 20 years we have witnessed a revolution in the management of combat polytrauma towards a goal of zero preventable battlefield death. Specialists from diverse disciplines challenged assumptions, collected and analysed data, conducted actionable research, made incremental care changes, measured outcomes and then repeated this cycle over and over again. In the end, new products were fielded, new techniques refined and organisational innovations realised. Several thousand lives were saved and combat casualty care was rapidly modernised.2–5 Our multidisciplinary team at the INOVA Heart and Vascular Institute aims for similar success defeating our own enemy: CS.
Cardiogenic Shock
CS, ‘the rude unhinging of the machinery of life’, is a state of end-organ dysfunction, often complicated by a systemic inflammatory response syndrome, secondary to insufficient cardiac output despite adequate preload, as a result of left ventricular (LV), right ventricular (RV), or biventricular (BiV) dysfunction.6–9 This complex and often multifactorial pathophysiological process is defined by haemodynamic parameters – systolic blood pressure <90 mmHg, cardiac index <1.8 litre/min/m2 without pharmacological support (or >2.2 litre/min/m2 with support), LV end-diastolic pressure >18 mmHg or RV end-diastolic pressure >10–15 mmHg or pulmonary capillary wedge pressure (PCWP) >15 mmHg – and clinical signs and symptoms of hypoperfusion, such as cool extremities, decreased urine output, and altered mental status.9,10
Following the uniform adoption of early revascularisation for acute MI (AMI), mortality rates for AMI CS decreased from near 90 % to <50 %.11–14 In the decades since, in-hospital survival rates have plateaued while the incidence of AMI CS and acute decompensated heart failure (ADHF) CS has increased despite improvements in door-to-balloon times (the cardiovascular specialist’s version of the surgeon’s ‘Golden Hour’) and adjunctive pharmacotherapy.15–28 Early survivors also suffer unacceptably high rates of post-discharge heart failure, rehospitalisation and death.29–32
Revascularisation is necessary but not sufficient for survival in AMI CS. Contemporary meta-analyses suggest no survival benefit to an immediate multivessel percutaneous coronary intervention (PCI) strategy compared with culprit vessel revascularisation in CS.33,34 Most recently, the randomised CULPRIT-SHOCK trial demonstrated a 7.3 % reduction in all-cause mortality rate at 30 days with a culprit-lesion-only PCI strategy versus immediate multivessel PCI in patients presenting with CS found to have multivessel coronary artery disease on angiography.25
Paradigm Shift
The fragility of critically ill patients with CS and multisystem organ dysfunction leaves little margin for error. The short-term stabilising effects of inotrope and vasopressor therapy are offset by adverse effects on afterload, oxygen demand, impaired tissue microcirculation, and arrhythmogenicity – translating into cardiotoxicity, end-organ dysfunction and higher mortality rates.35–40 The advent of rapidly deployable, user-friendly percutaneous mechanical circulatory support (MCS) devices may drive a paradigm shift in the treatment of CS: administration of circulatory and ventricular support to restore stable haemodynamics, minimise myocardial ischaemia, reduce native heart workload and maintain vital organ perfusion (Figure 1).
Previous preclinical investigations demonstrated haemodynamic benefits to early LV unloading and initiation of ventricular and circulatory support for ADHF CS and AMI CS.36,41–49 More recent studies suggest LV unloading may reduce reperfusion injury, myocyte loss and myocardial infarct size, and activate cardioprotective mechanisms preventing adverse remodelling.50–53 This approach – similar to the ‘damage control’ strategies employed by combat trauma surgeons – prioritises normal physiology over normal anatomy to prevent cardiovascular collapse and lethal multiorgan dysfunction.5
The current clinical evidence in favour of MCS employment to combat CS consists only of observational data, meta-analyses and small feasibility trials. While these investigations demonstrate superior haemodynamics and improved organ perfusion with percutaneous MCS employment in AMI CS, they do not demonstrate any survival benefit with this strategy.54–59
More recently, small single-centre studies (most notably the Detroit Cardiogenic Shock Initiative), international registry data and our own local experience lend some support to the immediate haemodynamic and potential short-term clinical survival benefits of initiation of percutaneous axial flow LV to aorta support as soon as possible after the onset of shock.36,56,60–66 Other investigations, such as the recent IMPella versus IABP Reduces mortality in STEMI patients treated with primary PCI (IMPRESS) trial, suggest that the benefits of percutaneous MCS devices are time-dependent and unlikely to impact outcomes if employed late, once overt multiorgan dysfunction has occurred.67
Team Building
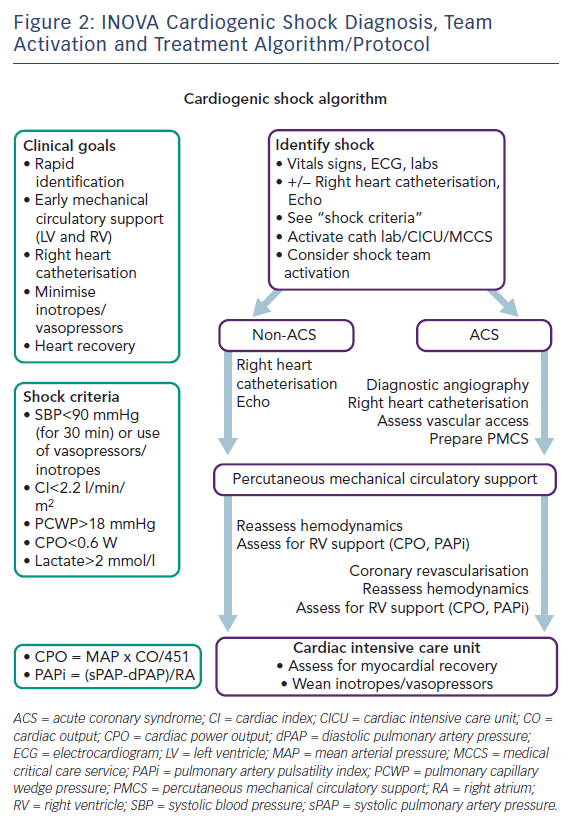
The INOVA Heart and Vascular Institute Cardiogenic Shock Initiative began in mid-2016 with the assembly of a diverse task force of clinical and administrative stakeholders across multiple disciplines to assess the current state of affairs, establish priorities of effort and assign ownership for these priorities.68–71 A detailed care pathway was proposed based on available scientific evidence. Graphics of our management algorithm (Figure 2) were posted in key work locations and laminated pocket cards distributed to hospital staff. Simultaneously, a 6-month-long training process focused on individual and team CS management skills, haemodynamic expertise, percutaneous MCS device insertion and management, team communication and dedicated protocol training. At the conclusion of our training and rehearsals, on 1 January 2017, the INOVA Cardiogenic Shock Team went live.
The INOVA Pathway
Our team selected five key areas of focus: rapid identification of shock (with early activation of our multi-specialty Shock Team and rapid collaborative decision making), early right heart catheterisation (to facilitate invasive haemodynamic-tailored therapy), expedited initiation of percutaneous MCS as appropriate (followed by early escalation as necessary), minimisation of vasopressor and inotrope use, and most importantly, meaningful patient recovery and survival.
The initial task of our team is the rapid identification of the shock state and assessment of its clinical severity via integrated clinical, laboratory, haemodynamic and imaging data.7,9,72 Immediate bedside echocardiography is used to assess cardiac function and identify potential causes of CS.73–77 While the indiscriminate use of right heart catheterisation in all-comers in the intensive care unit has proven ineffective, such detailed invasive haemodynamic data are essential for optimal management of CS, particularly when percutaneous MCS devices are used.78–84 In addition to typically measured parameters such as right atrial (RA) pressure, PCWP, systemic vascular resistance and cardiac output/cardiac index, our INOVA protocol further emphasises measurement of cardiac power output (CPO), RA:PCWP ratio and pulmonary artery pulsatility index (PAPi), all of which have recognised diagnostic and prognosticative power in the CS population.85–89
Protocol Implementation
Since initiating our INOVA cardiogenic shock programme in January 2017, there have been 161 team activations for AMI CS, ADHF CS and suspected or undifferentiated CS. Team activation occurs 24 hours per day, 7 days per week via a one-call process to a central operator at our Heart and Vascular Institute who gathers our five-person multidisciplinary team via either in-person or virtual (telephonic) bedside consultation. A consensus plan of care based on our protocol and established care priorities is developed and tailored to the specific clinical scenario.
In the cardiac catheterisation laboratory, we focus our institutional priorities on provision of axial flow percutaneous circulatory support and ventricular unloading prior to coronary reperfusion. For non-AMI aetiologies of CS, patients may instead require extracorporeal life support or urgent cardiac surgery. Decisions regarding sufficiency of support, need for escalation of support, and addition of right-sided or oxygenation support are made based on mandatory echocardiography and invasive haemodynamic reassessment prior to departing the bedside, angiography suite or operating room.
Although our protocol prioritises axial flow LV aortic assist devices, extracorporeal membrane oxygenation (ECMO) is also commonly used at our centre in cardiac arrest, respiratory arrest and severe BiV shock requiring higher levels of circulatory support – usually with concomitant LV unloading to mitigate the deleterious effects of increased afterload.90–93 Not infrequently, patients may require various ‘plug-and-play’ combinations of device support – such as Bi-Pella (combined left- and right-sided Impella® axial flow catheter support) or EC-Pella (combined ECMO and left-sided Impella support) to overcome the limitations inherent to each device.93–99
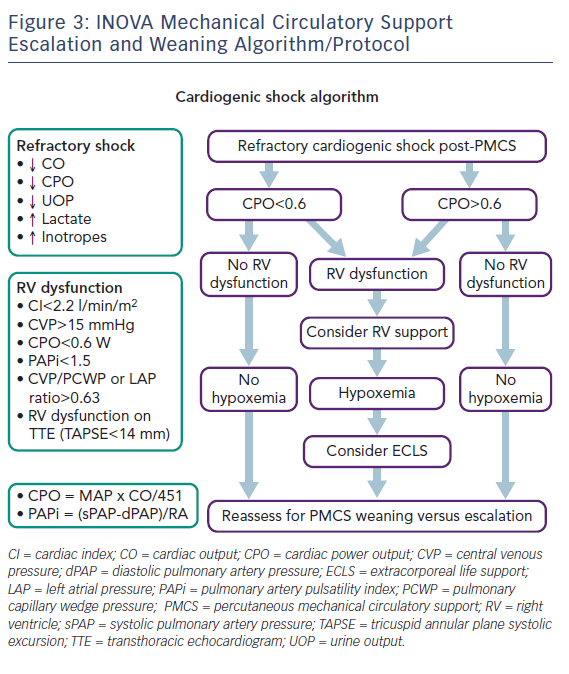
In the cardiac or cardiothoracic surgery intensive care unit, patients are co-managed by a co-attending team of an intensivist and a cardiologist or a cardiac surgeon providing collaborative 24-hour care.100 Joint rounds are conducted daily in conjunction with other multispecialty consultants. Serial physical exams are performed; lactate levels, organ function markers and urine output are repeatedly measured; bedside echocardiography is performed and invasive haemodynamics are regularly assessed. This continuous tracking of standard haemodynamic parameters as well as CPO and PAPi facilitates our team’s decisions regarding MCS escalation, addition of right-sided cardiac support and device weaning (Figure 3). This pattern of assessment, adjustment, reassessment and readjustment mirrors the ‘unblinking eye’ of continuous cyclic battlefield intelligence collection, analysis and dissemination.68,101
Between 1 January 2017 and 28 February 2018, our team managed 161 patients with CS: 41 % (n=66) AMI CS and 59 % (n=95) ADHF CS. Average patient age was 61 years (64 years for AMI and 59 years for ADHF). A total of 70 % of patients were male (n=112) and 30 % were female (n=49). Our initiatives to date have resulted in progressively improving outcomes for AMI CS and ADHF CS with in an increase in all-comer survival rates at our institution from 47 % (n=110) in 2016, to 61 % (n=140) in 2017, and 81 % (n=21) in the first 2 months of 2018.102 These data support our hypothesis that team-based multidisciplinary care, haemodynamic guidance and early consideration of MCS improve survival in patients with AMI or ADHF CS.103 Our single-centre 18-month results (to include outcomes by shock aetiology, patient age, initial haemodynamics and time to treatment) will be reported in late 2018.
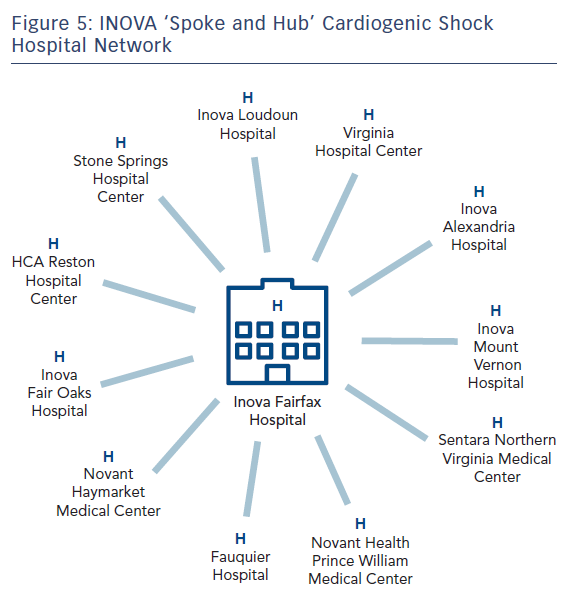
Even when successful, these aggressive team interventions are costly and labour-intensive and may not be suited to facilities without 24-hour on-site multidisciplinary cardiac, surgical and critical care services and advanced heart failure therapies, such as permanent ventricular assist device and cardiac transplantation. Such hospitals are therefore better served partnering with larger institutions as part of a ‘spoke and hub’ model.104–108
After Action Reviews
Our cardiogenic shock team conducts novel cross-discipline meetings with clinical and non-clinical staff and leaders with 100 % case review. Data related to each shock patient (i.e. clinical presentation, objective data, hospital course, clinical outcomes) are collected and reviewed every 2 weeks. We modified the validated military after action review model, which was designed to critique training and combat events and answer four questions: What was planned? What really happened? Why did it happen? What can we do better next time? (Figure 4).109 Our roundtable process assesses compliance to our protocols, determines the effectiveness of our interventions and facilitates regular incremental changes in our care pathways as part of a continuous process improvement programme.
Future Perspective
Looking forward, in our region and across the US, new networks of partnered multidisciplinary care ought to emerge on a large scale to establish linked regional systems of community hospitals and large centralised centres of excellence emphasising rapid triage, immediate transport and expedited door-to-support for patients with CS, emulating the highly successful military and civilian trauma systems and the cardiovascular communities historical successes in early revascularisation for acute ST-elevation MI.1,104–108 Locally, having focused on our ‘hub’ medical centre (Figure 5) in 2017, we are currently expanding our protocol and process to our linked ‘spoke’ hospitals (both internal and external to our health system) in 2018.
Due to the heterogeneous patient population and multifactorial aetiologies of death in CS, demonstrating a survival benefit will be challenging. However, ongoing treatment analyses (and hopefully rigorously performed randomised controlled trials) may continue to improve our understanding of modes of death in CS, identify ongoing areas for performance improvement and inform future guideline development.66,103,110–114
Conclusion
Although hampered by small sample sizes and lack of long-term outcomes data, current registries and single-centre reports, to include our own preliminary experience to date, suggest that team-based multidisciplinary care, early initiation of MCS, and haemodynamic-guided therapy may form the next leap forward in CS care to interrupt the vicious triad of ischaemia, hypotension and myocardial dysfunction and allow for myocardial salvage and meaningful patient recovery. In our first year, our INOVA team has worked to develop a heightened awareness of CS and an organisational commitment to building a comprehensive system of CS care, research and innovation, focused on our combat medicine inspired goal of zero preventable death from CS. We hope other institutions will do the same.