Dr Price began by presenting clinical data on imaging utilisation from the Scripps Clinic from 2013 to 2015. The usage of FFR has increased by approximately 50 % during these years, while the use of IVUS has fallen. In general, FFR is used in diagnostic procedures and IVUS is used to guide treatment.
A number of practical considerations need to be taken into account regarding the broad clinical application of FFR. First is the occurrence of technical errors. Pressure sensor drift can lead to falsely high or low FFR values. Falsely high FFR can result from equalisation with the guide not sufficiently being disengaged, inadequate hyperaemia or deep seating of the coronary guide catheter. On occasions, it is impossible to perform FFR due to an inability to deliver the wire distal to the target lesion. In addition, operator frustration can occur since pressure wires do not have the performance characteristics of workhorse coronary wires. Challenges associated with the use of pressure wires include wire kinking or lack of support when advancing or removing balloons and stents; the need for multiple pull-backs and rewiring for tandem lesions. These challenges contribute to long procedural times, particularly for cases involving more than one vessel.5 Using FFR in patients with multivessel disease requires multiple advances and withdrawals and therefore durable wires are needed. A coronary workhorse wire is often needed in addition to pressure wires in these cases.
Evidence in support of the use of FFR comes from the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) study, a multicentre trial in which patients (n=1,005) with multivessel coronary artery disease (CAD) were randomised to undergo angiography-guided PCI or FFR-guided PCI. Recent 5-year data confirms the long-term safety of FFR-guided PCI.6 A study of patients enrolled in the FAME study found that a FFR-guided SYNTAX score (SS), termed ’functional SYNTAX score‘ (FSS), would predict clinical outcome better than the classic SS in patients with multivessel CAD undergoing PCI. Using FFR, 32 % of patients with a high SS shifted to the lower-risk group (15 % of high and 59 % of intermediate shifted to low).7
Two types of FFR technologies are commonly employed in clinical practice. Pressure wire technology involves a specially constructed 0.014" wire. A pressure sensor is incorporated into the distal end at the junction of the radiopaque and radiolucent sections. Piezoelectric technology is most commonly used, which has inherent problems, including pressure drift. In addition, the performance of pressure wire technology is not as robust as a dedicated workhorse coronary wire. Microcatheter technology employs a low-profile catheter with a pressure sensor that employs fibre-optic technology incorporated into the distal end. It can be delivered over a standard 0.014" coronary wire.
The RXi Rapid Exchange FFR technology features a marker band located 2.5 mm from tip, a fibre-optic sensor 2.5 mm proximal from the marker band (5 mm from the tip), tip diameter 0.016" and a profile comparable to 0.022" diameter at the lesion site. This system has the potential to overcome some of the limitations associated with conventional pressure wire systems. Equalisation of the catheter is a straightforward procedure: the guide is disengaged from the ostium, the catheter sensor positioned distal to the guide tip and proximal to left main ostium and the guide flushed with saline. The distal workhorse wire facilitates guide catheter disengagement during maximal hyperaemia and re-engagement once complete. There is also no risk of losing the guide position, unlike with a pressure wire, where even gentle orientation can cause the position to be lost.
Tandem lesion assessment is very common and can be quite laborious with a pressure wire. The microcatheter FFR can help evaluate which target lesion is functionally significant. For example, using a pressure wire, the wire is advanced into the distal vessel. Next, wire pullback is performed across lesions during hyperaemia. The lesion that is associated with the greatest absolute drop in FFR is rewired and the necessary intervention performed. Pullback is repeated, then rewiring and interventions are performed on the remaining lesions as needed according to FFR. Repeat pullbacks and advancements, as well as multiple wires, are needed when performing interventions across stents and multiple plaques. The microcatheter FFR system, in which the tandem lesions are wired once with a workhorse wire, improves workflow and reduces the risk of multiple rewiring of target lesions that would be required if one used a pressure wire.
Unresolved questions remain in catheter-based FFR. It is not known whether drift is improved with the fibre-optic sensor of the Navvus compared with pressure wires. It is also uncertain whether the volume taken up by the catheter within the vessel can influence the pressure ratio (Pd/Pa) and FFR. In turn, we do not know whether the diameter of the vessel and at the lesion site affects the diagnostic accuracy of the Navvus catheter compared with a pressure wire.
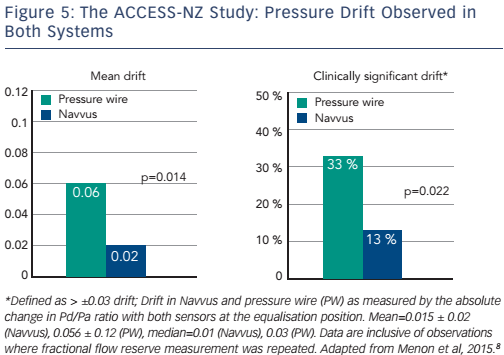
Some clinical data are available to address these questions. The ACIST Diagnosis of Coronary Arterial Disease with a Rapid Exchange Monorail Pressure Sensor (MSP) for the Measurement of Fractional Flow Reserve (FFR) (ACCESS-NZ) study (n=58) evaluated the safety and efficacy of the Navvus catheter to assess coronary FFR, compared to a pressure wire system. There was less sensor drift with the optically based Navvus catheter than with the pressure wire (0.02 ± 0.02 Navvus versus 0.06 ± 0.12 pressure wire, p=0.014). In addition, fewer patients had clinically significant (p≥0.03) drift with the Navvus catheter (13 %) than with the pressure wire (33 %; see Figure 5). No significant mean difference was reported between FFR measured by microcatheter compared to pressure wire acquired FFR. Within the measurement uncertainty of the pressure wire FFR, in no cases was the diagnostic assessment by Navvus different than that of the pressure wire.8
The Assessment of Catheter-based Interrogation and Standard Techniques for Fractional Flow Reserve Measurement (ACIST-FFR) prospective, open label study aims to recruit 240 patients at 12 US sites.9 Enrolment began in November 2015. The study’s objective is to assess the differences, if any, between FFR measured by the Navvus catheter and another commercially available 0.014" pressure guidewire (St Jude or Volcano) in subjects with CAD undergoing coronary angiography. It aims to estimate bias with a narrower confidence interval (CI) than in the ACCESS-NZ study, include quantitative vessel measurements and, importantly, extend the inclusion criteria to smaller vessels, including any vessel in which a stent implantation is planned.
The general inclusion criteria are broad: age 18 years or older; a clinical indication for coronary angiography; and that the subject or subject’s legal representative has the ability to understand and provide signed consent for participating in the study. Angiographic inclusion criteria are a vessel with a thrombolysis in myocardial infarction (TIMI) flow of 3; a de novo lesion that a physician has considered to have a clinical indication for FFR measurement; and operator-assessed reference vessel diameter of the target lesion ≥2.25 mm. General exclusion criteria are acute ST-elevation or non-ST-elevation MI as the indication for coronary angiography and New York Heart Association (NYHA) Class 4 severe heart failure. Angiographic exclusion criteria are target vessel with an angiographically visible or suspected thrombus; target lesion within a bypass graft; angiographic evidence of a dissection prior to initiation of pressure wire measurements; and target vessel containing excessive tortuosity or calcification. The primary endpoint is the correlation between Navvus and pressure wire FFR measurements, as assessed by the Bland–Altman analysis. Secondary endpoints include slope of and passing-Bablok Fit; diagnostic accuracy using pressure wire FFR ≤0.80 as standard; device success rate; mean drift, defined as the absolute difference between Pd/Pa at the equalisation position after pullback and 1.00, for each system individually and comparison between the two systems; rate of drift; and rate of device-related adverse events.
The use of intracoronary contrast to induce hyperaemia for FFR is also currently being investigated. A recent multicentre study (n=763) compared the diagnostic performance with adenosine-derived FFR, ≤0.80 of contrast-based FFR, Pd/Pa and the instantaneous wave-free ratio (iFR). The study found that contrast FFR provided diagnostic performance superior to that of Pd/Pa or iFR for predicting FFR (85.8 % accuracy versus 78.5 % and 79.9 % for Pd/Pa and iFR, respectively. The investigators concluded that contrast FFR was easy, inexpensive and safe, and displayed excellent test/retest stability.10
In summary, with increased use of FFR, particularly in multivessel disease and tandem lesions, convenience and accurate technique are important. Catheter-based FFR is easy to use and helps avoid technical errors. Contrast FFR together with catheter-based FFR is a quick and painless procedure. The prospective, multicentre ACIST-FFR study will address unanswered questions about this technology.