Dr Hajjar is the Director of the Cardiovascular Research Center and the Arthur and Janet C Ross Professor of Medicine at the Mount Sinai School of Medicine. His research focuses on molecular mechanisms of heart failure (HF) and his team has validated the cardiac sarcoplasmic reticulum calcium ATPase pump (SERCA2a) as a target in HF, developed methodologies for cardiac-directed gene therapy, examined the functional consequences of SERCA2a gene transfer in the failing heart, and conducted first-in-man clinical trials testing the efficacy of gene transfer in patients with HF. Dr Hajjar is a founding member of the A-CURE Working Group.
Dr Hajjar began by examining the role of gene therapy in HF. He drew a distinction between cellular therapy, which allows the introduction of new cells that can help the remodelling of damaged or diseased myocardium or extracellular matrix, and gene therapy, which focuses on altering the function of diseased cardiac cells at the level of the single gene. In the last decade there has been invigorated interest in cardiac gene therapy as a result of increasingly efficient gene transfer technologies and safer vectors that allow the homogeneous transduction of cardiomyocytes. Critical advances that have supported the increased use of gene therapy include the ability to induce long-term expression of the target gene, viral vectors with higher cardiac specificity and minimally invasive vector delivery techniques.1,2 Dr Hajjar’s team is investigating gene replacement therapy using adeno-associated virus (AAV) vectors delivering the SERCA2a gene. These vectors have been demonstrated to be safe and non-pathogenic; the majority of the population has been exposed to the wild-type virus in childhood without any evidence of disease.
The efficiency of gene transfer is has been a major obstacle to the successful translation of gene therapy into the clinic. The rate of in vivo viral transduction reported in clinical trials is too low to induce any physiological impact. The efficiency of gene transfer to the heart can be improved by increasing perfusion pressure, coronary flow, vector dose, and dwell time. The preferred method of administering the vector is through percutaneous intracoronary artery infusion, since this approach more readily ensures gene delivery to the viable myocardium. The Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) clinical trials investigated this method of intracoronary administration of AAV type 1 (AAV1)/SERCA2a in patients with Class III/IV HF. CUPID 1 was a randomised, doubleblind, placebo-controlled, Phase IIa study in patients with advanced HF. Following the administration of intracoronary AAV1/SERCA2a or placebo, significant increases in time to clinical events and decreased frequency of cardiovascular events were observed at 12 months in the treatment group (hazard ratio=0.12; P=0.003), and mean duration of cardiovascular hospitalisations over 12 months was substantially decreased (0.4 versus 4.5 days; P=0.05) on high-dose treatment versus placebo.3
The follow-up and larger Phase IIb study (CUPID 2) is the largest gene transfer study carried out in humans to date (n=250). However, AAV1/SERCA2a at the dose tested did not show an improvement in the primary endpoint.4 Possible reasons for this disappointing result include insufficient myocardial uptake, because the AAV concentration was too low (the US Food and Drug Administration did not allow the use of higher doses), and the method of gene transfer was inadequate. While previous data in animals had showed a high percentage of infected cardiomyocytes (30–75 %), data from CUPID 2 showed that the uptake in humans was much lower (<0.5–1 %). The method of gene transfer in CUPID 2 trial was clearly inadequate.
Dr Hajjar presented his current hypothesis that involves improving gene delivery by using the Impella device to enhance viral uptake. He proposed that Impella support could affect uptake in two ways. First, viral uptake is adversely affected by increased left ventricle (LV) diameter, end diastolic pressure and sympathetic activation, leading to increased wall stress. Further, the inflammation, cell death, ischaemia and myocyte destruction at the time of a myocardial infarction (MI) also provides a hostile environment for vectors. Acute unloading with the Impella mitigates these adverse conditions. Second, the Impella could be used to haemodynamically support the patient while the vector is delivered into the coronary system during temporary coronary balloon occlusion. This would allow for a longer dwell time and minimise the risk of haemodynamic collapse.
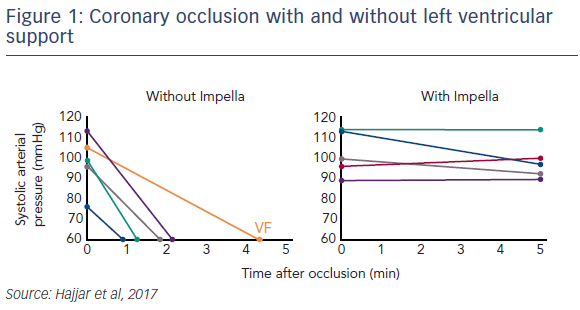
Dr Hajjar presented data from his current studies. In a porcine model of subacute ischaemic HF, MI is induced, and the heart is allowed to remodel for 2 weeks. Gene delivery under Impella support then commences at this time point. Early data shows that this approach reduces LV wall stress, decreases end diastolic pressure, increases epicardial coronary flow, and increases myocardial perfusion, specifically in the infarct region (see Figure 1).5 Thus far, all pigs receiving Impella support during vector delivery while occluding the coronary artery have been successfully bridged through the procedure without incident, while all pigs that did not receive mechanical support suffered haemodynamic collapse and required cardioversion or other intervention.
In conclusion, these ongoing studies hope to demonstrate that by enhancing coronary flow, perfusion pressure can be increased while at the same time the unloading will allow a better environment for more aggressive gene delivery.