Dr Kapur’s talk covered the new and rising areas of research interest in the field of ventricular unloading.
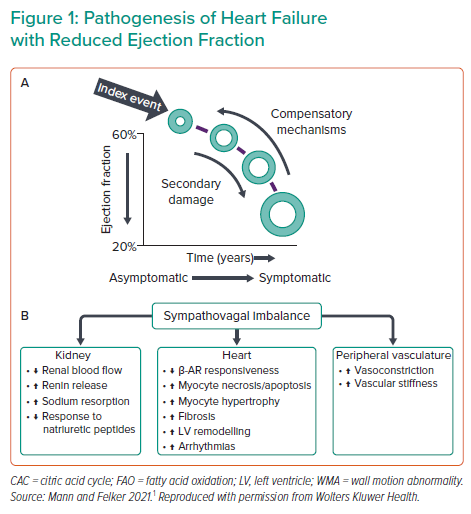
In the field of cardiac unloading, emerging research topics include heart failure and heart recovery. Despite advances in drug and device therapies, the prognosis for heart failure is poor and the disease burden is increasing in the US. This continued clinical need provides the rationale for gaining a better understanding of the physiology and biology of heart failure and remodelling. Research efforts have uncovered many molecular players in the myocardial response to damage and compensatory mechanisms.1 Dr Kapur noted that the big question is whether the damaged heart reverse remodel back in the current era (Figure 1).
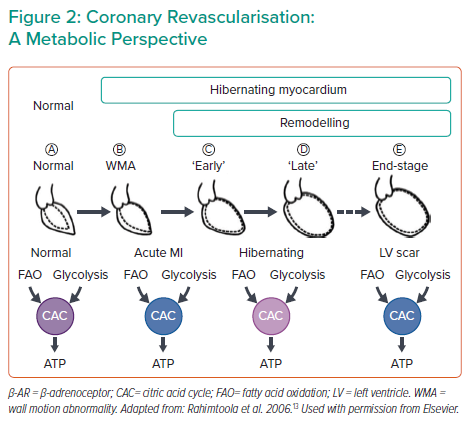
Another topic in the field is the question of elective coronary revascularisation. In patients with acute MI (AMI) presenting with or without shock, revascularisation is a must to rescue the myocardium from ischaemia. However, recent surgical and percutaneous coronary intervention (PCI) literature has reported that patients with ischaemic cardiomyopathy (ICM), early or late stage, may also benefit from revascularisation. From a metabolic perspective, the normal myocardium prefers fatty acid oxidation and oxidative phosphorylation to generate ATP, whereas myocardium under ischaemia has to shift to inefficient glycolysis (Figure 2), limiting its recovery potential. The other key factor in this setting is that adverse cardiac remodelling is load dependent: analysis of large randomised control trials showed that reduced left ventricular (LV) end systolic volume is indicative of favourable clinical outcomes in revascularisation, suggesting that load reduction is beneficial in ischaemia.2 Studies are underway to evaluate whether unloading during revascularisation in AMI or ICM additionally promotes myocardial recovery.
Bringing these concepts together, the ultimate question for the cardiac unloading community is whether acute cardiac unloading can be harnessed as a therapeutic approach to improve myocardial recovery. It will be important to test unloading as a therapeutic strategy beyond its current role as an enabler of adjustive procedures, in particular in reversible damage settings such as AMI.
The concept of unloading as a therapy is supported by historical haemodynamic science, going back to Drs Frank and Starling in the 1800s. Fundamental studies in haemodynamics demonstrated that myocardial oxygen consumption is proportional to mechanical work, and calcium cycling and basal metabolism constitute basal oxygen consumption.3,4 The advent of LV assist devices in the 2000s presented a major paradigm shift in the field of cardiac replacement and recovery. This was the beginning of a shift to understanding how reduced pressure–volume area and work aid in heart recovery. As a result, there are now a range of opportunities to intervene with unloading in the clinical setting, including AMI with or without cardiogenic shock (CS), high-risk PCI, acute decompensated heart failure (ADHF), ADHF with or without CS and advanced HF.
The concept of reducing myocardial oxygen demands in AMI was first introduced in 1971.5 That seminal study demonstrated that, in addition to restoring the blood flow, reducing myocardial oxygen demand further reduced the infarct scar and damage. Ventricular load in ischaemia refers to any variable that increases myocardial oxygen demand, such as heart rate, LV wall stress, LV systolic and diastolic pressures and stroke work. These variables are now being considered through the lens of unloading, and many interesting studies are underway.
Most cardioprotective approaches in MI have historically focused on reducing reperfusion injury, but new approaches are investigating how reducing ischaemic burden can change the trajectory of myocardial damage. The concept that ‘time is muscle’ is extremely important; however, it is also important to recognise that unloading may now create a window in the time trajectory to allow other strategies of intervention.
The development of transvalvular pumps, such as Impella, has expanded the field of ventricular unloading. One of the earliest mechanistic studies with Impella showed that the mechanism of myocardial salvage involved not only increased myocardial perfusion, but also a reduction in metabolic requirements.6 In that study, Meyns et al. showed that reduced myocardial oxygen consumption under unloading was correlated with reduced infarct scar. This occurred not only during the reperfusion phase, but also during the ischaemic phase, uncoupling ischaemia from reperfusion.6
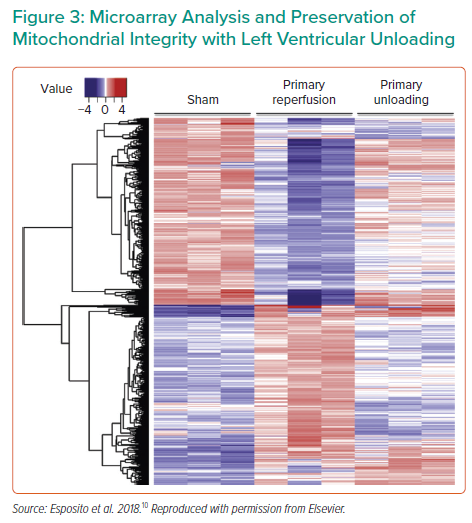
The timing of unloading is another important research topic. A number of ongoing studies are investigating the timing of unloading with regard to reperfusion, as well as the impact of unloading on coronary blood flow. For example, recent work showed that Impella unloading prior to reperfusion increases coronary collateral flow, which continues to increase over 30 minutes and optimises flow prior to opening a blocked artery.7 Dr Kapur and colleagues demonstrated that mechanical unloading for 30 minutes prior to reperfusion not only reduced infarct size, but also produced measurable changes in the cardioprotective signalling pathways;8 this was further improved by lengthening the unloading prior to reperfusion to 60 minutes.9 Dr Kapur’s group also performed transcriptomic profiling of the hearts after primary reperfusion versus primary unloading, identifying an array of differentially altered genes and pathways (Figure 3),10 which warrants continued investigation and therapeutic development. One of the major pathways altered was mitochondrial metabolism; genes related to mitochondrial integrity, as well as the physical structure, were protected in the primary unloaded hearts.10
This has highlighted the need to study the role of dysfunctional mitochondria in myocardial recovery. Hypoxia, and particularly the gene hypoxia inducible factor 1 (HIF-1), is a marker of the level of ischaemia in a tissue. Dr. Kapur noted the possibility that HIF1 may be a therapeutic target in ischaemia, as well as in unloading and myocardial recovery. Dr Kapur’s recent work showed that unloading promotes increases in myocardial HIF-1 expression; using molecular tools, his group showed that increased HIF-1 was necessary for reductions in infarct size with unloading.11 Furthermore, unloading increased HIF-1 expression even after reperfusion, suggesting that pharmacological manipulation of HIF-1, in tandem with unloading, could be a novel strategy in further reducing infarct size.
Finally, calcium cycling is another exciting new target for unloading research. Recent work has revealed that there are changes in calcium handling that occur days and weeks after a period of unloading, which is beginning to unravel the long-term implications of calcium cycling on myocardial recovery.12 A deeper dive into extracorporeal membrane oxygenation (ECMO) is also ongoing, because how ECMO affects right ventricular or LV biology, vascular biology or the kidney is currently poorly understood.
Dr Kapur closed by highlighting the great scientific achievements the A-CURE research community has brought forth over the years: many more exciting findings are to come.