Small vessel coronary artery disease (SvCAD) is a significant risk factor for adverse events in percutaneous coronary intervention patients. It is often diffuse and multivessel on presentation and confers worse outcomes with higher rates of major adverse cardiac events (MACE) and target lesion failure after intervention.1,2 Best practice guidelines on the management of SvCAD interventions remain limited.
Drug-eluting stents (DESs) were first described in 2002 and demonstrated reduced rates of in-stent restenosis compared with bare-metal stents, decreasing risk by 60–75% across lesion and patient subsets.3–6 However, DESs introduce a range of complications, especially when deployed in SvCAD.7 Their use is associated with delayed healing, inflammation and impairment of the endothelial function of the coronary vessel.8 This may increase the incidence of late and very late thrombosis, with one meta-analysis indicating a fivefold increase in late thrombosis compared with bare-metal stents.8–10 They also necessitate a longer duration of dual antiplatelet therapy, and while DESs have reduced the risk of in-stent restenosis, they do not remove the risk.4,11 Finally, despite their widespread use, the post-marketing analysis has often been limited to 1 year of follow-up, and the long-term and SvCAD clinical outcome data are limited.10
Drug-eluting balloons (DEBs) are a novel and evolving technology. They use a semi-compliant balloon catheter coated in a lipophilic antiproliferative drug. DEBs are currently recommended as class IA for treating in-stent restenosis. However, their use for other indications, including SvCAD, acute MI and bifurcating lesions, is less defined.12 Revascularising the vessel without leaving a foreign body provides an attractive alternative in treating SvCAD.
An important consideration in revascularisation trials is the duration of follow-up. The STICH trial comparing coronary artery bypass grafting with percutaneous coronary intervention did not show significant results until 2 years of follow-up. Furthermore, the SCAAR registry reported target lesion restenosis with DEB versus DES in de novo lesions did not differ until 6–12 months of follow-up.13,14 Finally, studies highlighting similar performance of optimal medical therapy with intervention in stable disease highlights the need for long-term safety data if performing coronary intervention.15
This paper aimed to perform a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) systematic review and meta-analysis of published randomised control trials comparing DES and DEB technologies for treating small coronary artery disease (<3 mm). We aimed to present the most comprehensive long-term follow-up data of published randomised control trials (RCTs) up to 3 years.
Methods
Search Strategy
A systematic review was performed of published literature following the PRISMA guidelines. Review protocol and registration were not used. Inclusion and exclusion criteria were as follows:
Inclusion Criteria
- Randomised control trial or RCT follow-up study with reporting of 1-year clinical outcomes or longer.
- Comparing drug-coated balloons and drug-eluting stents in acute and stable coronary artery disease.
- Reporting on small coronary arteries of <3 mm.
- English language.
Exclusion Criteria
- Non-randomised trial.
- Follow-up of outcomes <1 year.
- Those not comparing drug-eluting balloons and drug-eluting stents in small coronary arteries <3 mm.
- Those not available in the English language.
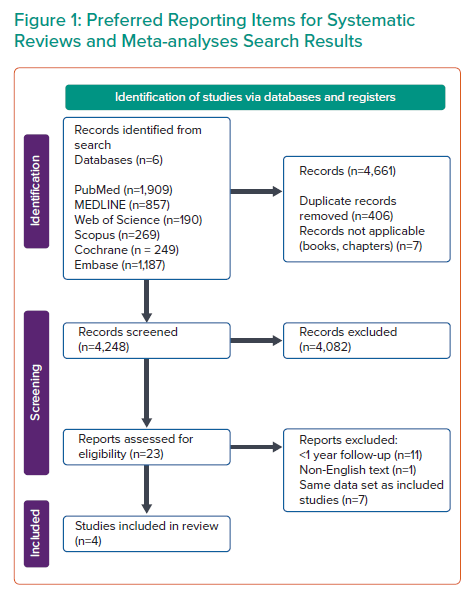
The Boolean operators ‘AND’ and ‘OR’ were applied to key search terms ‘drug-eluting balloon’, ‘drug-coated balloon’, ‘drug-eluting stent’, ‘drug coated stent’, ‘percutaneous coronary intervention’, ‘small coronary artery’, ‘small coronary vessel’, ‘small vessel’ and ‘randomized control trial’, and the Boolean operator ‘NOT’ was applied to ‘femoral’, ‘popliteal’ and ‘peripheral artery disease’. A range of databases was searched, including PubMed with 1,909 search results, Medline OVID with 857, Web of Science with 190, Scopus with 269, Cochrane with 249 and Embase with 1,187. All databases returned a total number of 4,661 articles as of August 2021. Search results are shown in Figure 1.
The results were screened for duplicates, with 406 identified. Seven books were removed, yielding 4,248 results. The titles of the results were screened as per PRISMA guidelines by two authors, GM and AN, and all disputes were resolved by consensus. A total of 166 studies were selected for review of abstracts, 23 were identified for full-text review and four met the inclusion criteria. These RCTs are BASKET-SMALL 2 (3-year follow-up study), PICCOLETO 2, BELLO (2-year follow-up study) and RESTORE SVD.16–19 Two conference abstracts on 2- and 3-year long-term outcomes for the included RESTORE SVD study were identified in the primary search and included (Figure 1).19–21
Outcome Measures
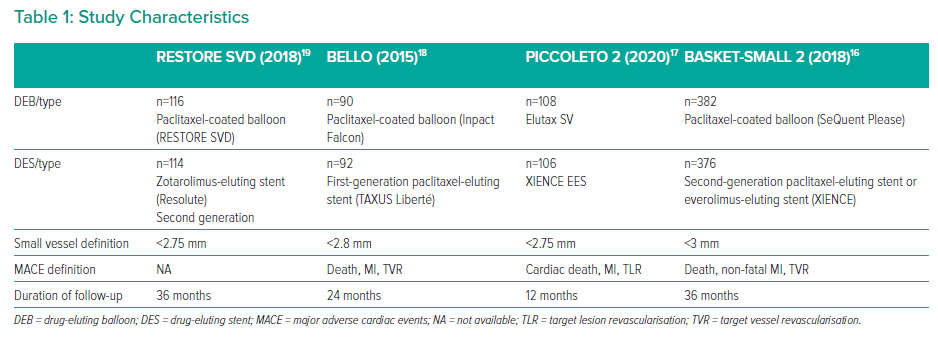
The primary endpoint was the long-term composite outcome of MACE, as outlined in Table 1. Secondary outcomes were all-cause mortality, myocardial infarction, cardiac death, target vessel revisualisation, vessel thrombosis, major bleeding, target vessel revascularisation, and target lesion revascularisation at 1, 2 and 3 years (Tables 1 and 2).
Data Extraction
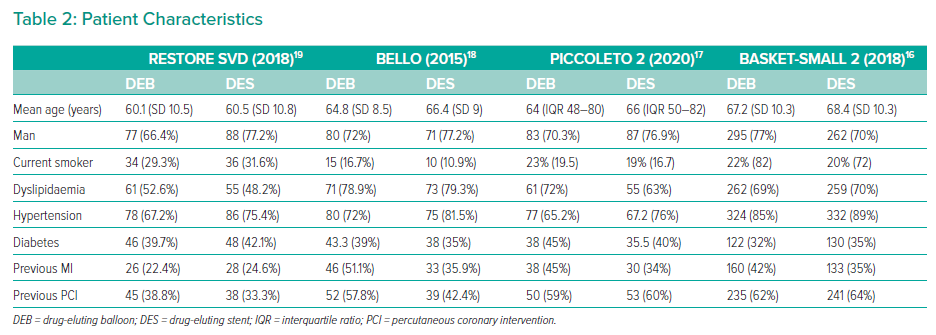
Data were extracted in line with Cochrane guidance independently by GM and AN, with primary and secondary outcome data beyond 1 year extracted. As shown in Tables 1 and 2, study and patient characteristics were identified. The RESTORE SVD trial subgroup of very small vessels <2.25 mm was included in the larger group, as both met the inclusion criteria of <3 mm.19
Statistical Analysis and Quality Analysis
Included studies were assessed for long-term clinical outcomes >1 year. Data were present for up to 3 years of follow-up. Statistical results were analysed using the Cochrane RevMan tool version 5.4. All outcomes were dichotomous and used the Mantel–Haenszel and random effects statistical and analytical model. The OR is presented with a 95% CI. For all results, “favours DEB” was identified as a shift to the left and “favours DES” as a shift to the right.
The I2 index assessed heterogeneity between the studies, with the predetermined cut-off of >50% for heterogeneity of statistical significance, as per Higgins et al.22 Sensitivity analysis was performed on all results by excluding each study in turn and assessing the robustness of the findings.
Risk of bias assessment was performed using the Cochrane risk of bias tool (ROB2) and RevMan 5.4, with GM and AN assessing each study for bias (Supplementary Material Figures 1 and 2). All studies demonstrated performance bias due to the interventional nature of the research. Across all other markers in the tool, the RCTs maintained a low or unclear risk of bias. (Supplementary Material Figures 1 and 2).
Results
The results of the systematic review are shown in Figure 1. Of 4,661 articles, four RCTs were included. A total of 1,414 patients were randomised in included trials. Study and patient characteristics are shown in Tables 1 and 2, respectively.
Major Adverse Cardiac Events
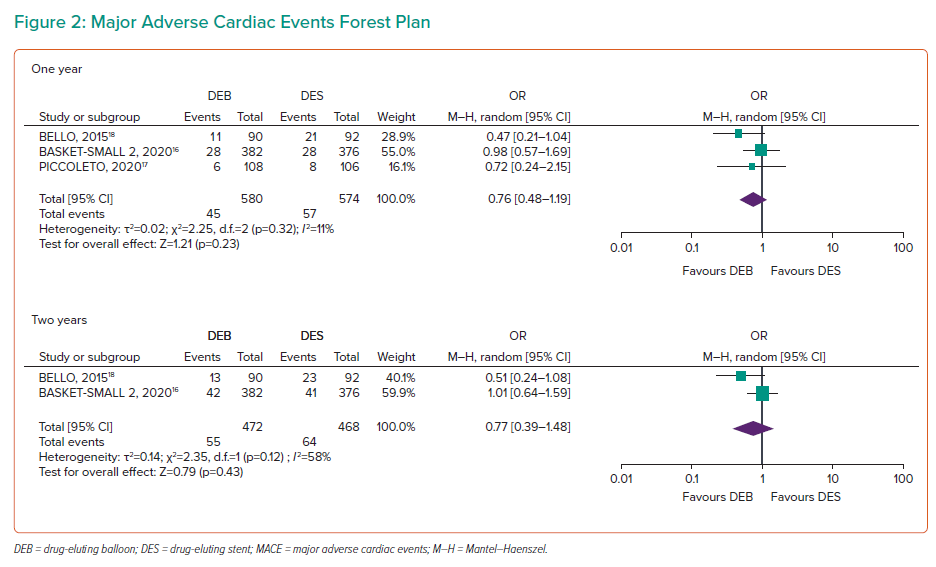
MACE at 1 year included 1,154 participants, indicating no significant difference between the two arms (OR 0.76; 95% CI [0.48–1.19]; Figure 2). At 2 years, 940 participants demonstrated no significant difference (OR 0.77; 95% CI [0.39–1.48]). BASKET-SMALL 2 reported 3-year data with no statistical difference in MACE (OR 0.98; 95% CI [0.65–1.48]).16 Heterogeneity was 11% at 1 year and 58% at 2 years, indicating moderate heterogeneity for 2-year data beyond the predetermined threshold.
All-cause Mortality
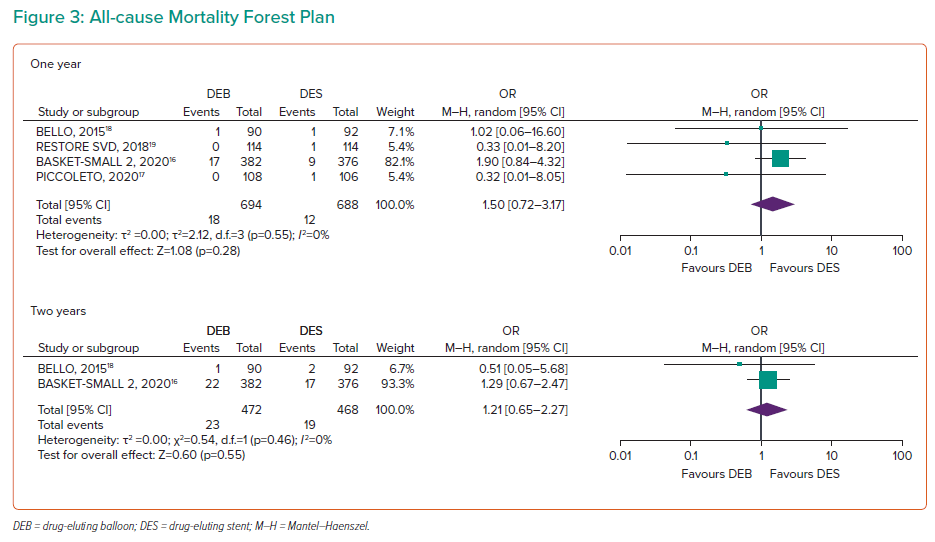
All four RCTs, including 1,414 participants, demonstrated all-cause mortality data at 1 year, with no significant difference between DEBs and DESs (OR 1.50; 95% CI [0.72–3.17]; Figure 3). Two-year data were available for BELLO and BASKET-SMALL 2, with no significant difference (OR 1.21; 95% CI [0.65–2.27]).16,18 Three-year data were available for RESTORE SVD (OR 1.02; 95% CI [0.59–1.77]).19 No heterogeneity was found for 1- and 2-year outcomes.
MI
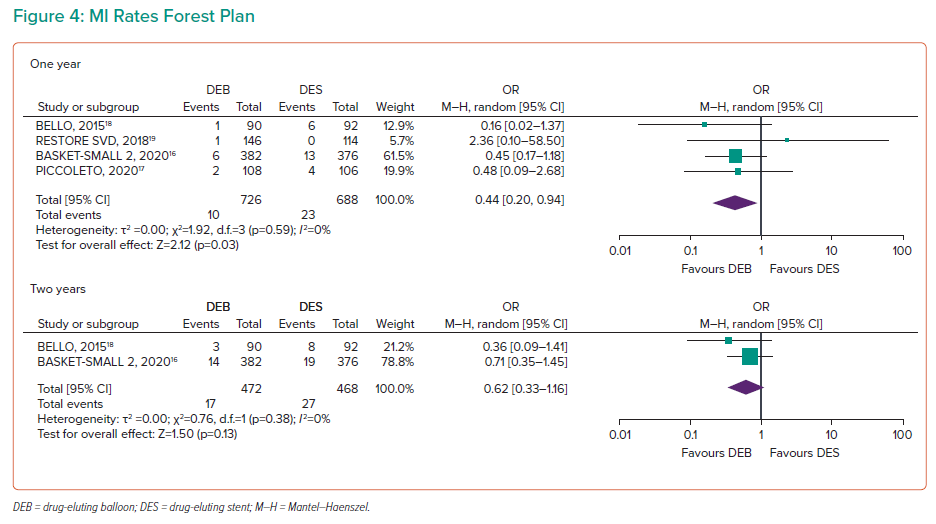
MI rates were presented in all four RCTs at 1 year, including 1,414 participants, indicating a significant reduction in MI for the DEB arm at 1 year (OR 0.44; 95% CI [0.2–0.94]; Figure 4). On sensitivity analysis, the difference became more significant if both RESTORE SVD and PICCOLETO 2 were removed from the analysis (OR 0.37; 95% CI [0.15–0.91]), and became non-significant when BELLO and BASKET-SMALL 2 were removed (OR 0.69; 95% CI [0.15–3.12]).16–19
Two-year data were available for BASKET-SMALL 2 and BELLO, indicating no significant difference (OR 0.62; 95% CI [0.33–1.16]), while BASKET-SMALL 2 recorded 3-year MI data with no significant difference (OR 0.8; 95% CI [0.43–1.5]).16,18
Cardiac Death
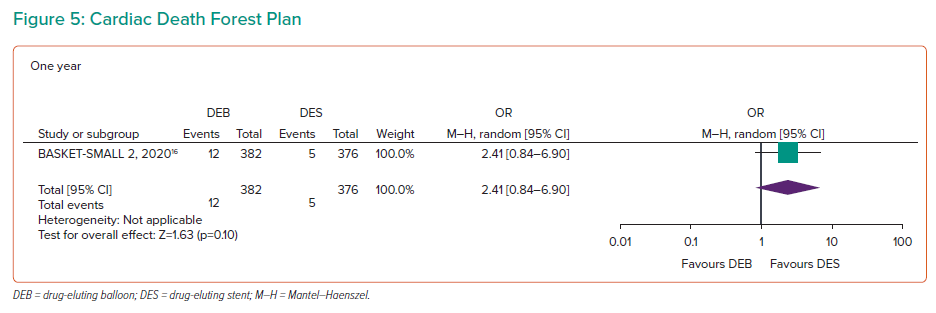
Cardiac deaths were recorded in all four RCTs with 1,414 participants at 1 year (Figure 5). However, no cardiac deaths occurred in any arms of the BELLO, RESTORE SVD or PICCOLETO trials.17–19 BASKET-SMALL 2 demonstrated events in both arms at 1 year (OR 2.41; 95% CI [0.84–6.9]), 2 years (OR 1.55; 95% CI [0.66–3.63]) and 3 years (OR 1.3; 95% CI [0.62–2.72]).16 No significant differences were found between the two study arms regardless of follow-up duration.
Vessel Thrombosis
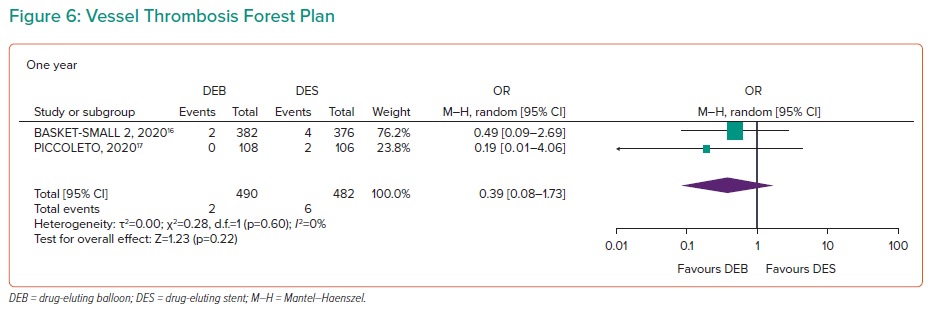
Vessel thrombosis was recorded in BELLO and BASKET-SMALL 2 at 1 year (1,232 participants), indicating no statistically significant differences between the two arms (OR 0.39, 95% CI [0.09–1.73]; Figure 6).16,18 The study heterogeneity was 0% at 1 year. BASKET-SMALL 2 further recorded data at 2 years (OR 0.32; 95% CI [0.07–1.62]) and 3 years (OR 0.32; 95% CI [0.07–1.62]), with no significant differences.16
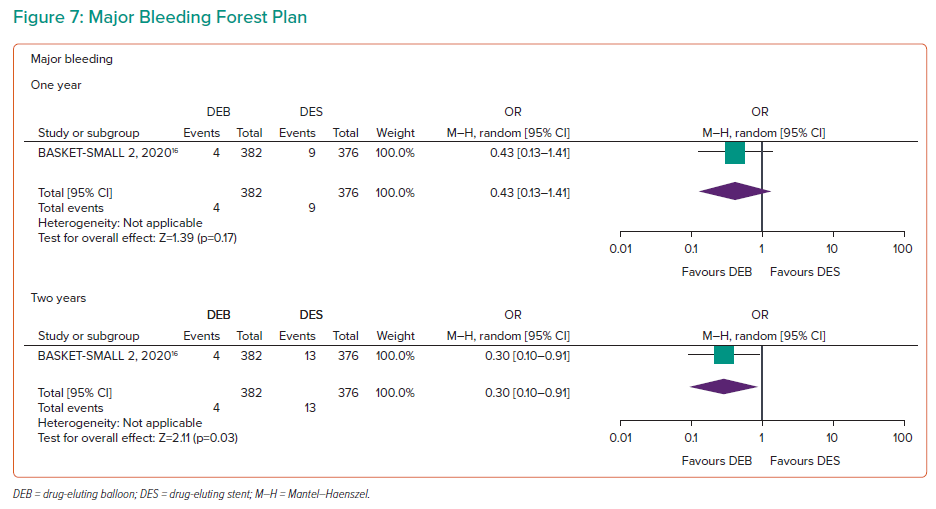
Major Bleeding
The PICCOLETO 2 and BASKET-SMALL 2 trials recorded data for 1 year with 972 participants (Figure 7).16,17 PICCOLETO 2 recorded no events at 1 year, and BASKET-SMALL 2 reported no significant difference between the arms (OR 0.43; 95% CI [0.13–1.41]).16,17 The 2- and 3-year follow-up data were recorded for BASKET-SMALL 2, with a statistically significant reduction in the odds of major bleeding at 2 years (OR 0.3; 95% CI [0.1–0.91]), with no difference at 3 years and a trend towards the DEB arm (OR 0.41; 95% CI [0.16–1.09]).16
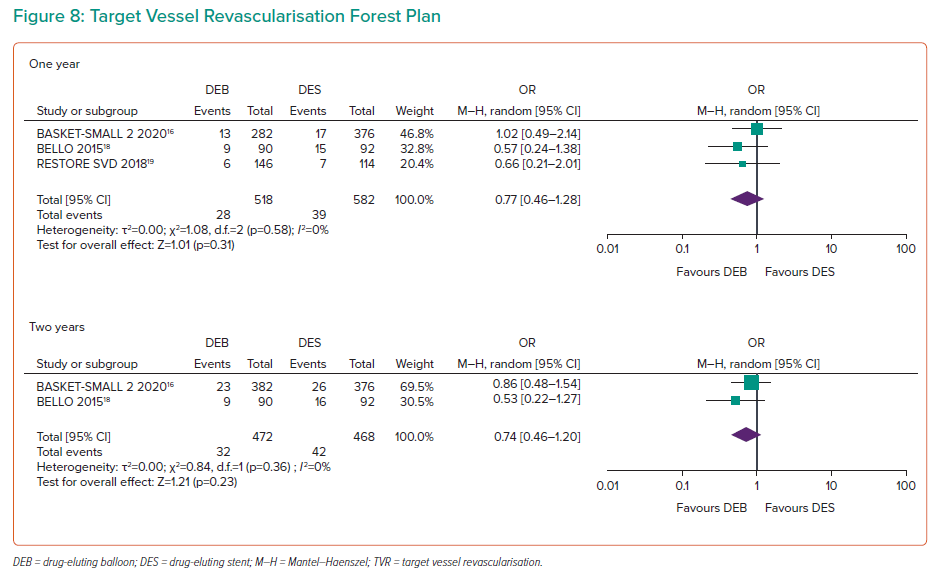
Target Vessel Revascularisation
RESTORE SVD, BELLO and PICCOLETO 2 recorded data on target vessel revascularisation (TVR) for 1,100 participants at 1 year of follow-up (Figure 8).17–19 TVR rates indicated no difference at 1 year (OR 0.77; 95% CI [0.46–1.28]). The 2-year data were presented for BELLO and BASKET-SMALL 2 (OR 0.74; 95% CI [0.46–1.2]), and 3-year data for BASKET-SMALL 2 (OR 0.92; 95% CI [0.54–1.54]), with no significant difference.16,18 The heterogeneity was 0% for 1- and 2-year data, with no change in the sensitivity analysis.
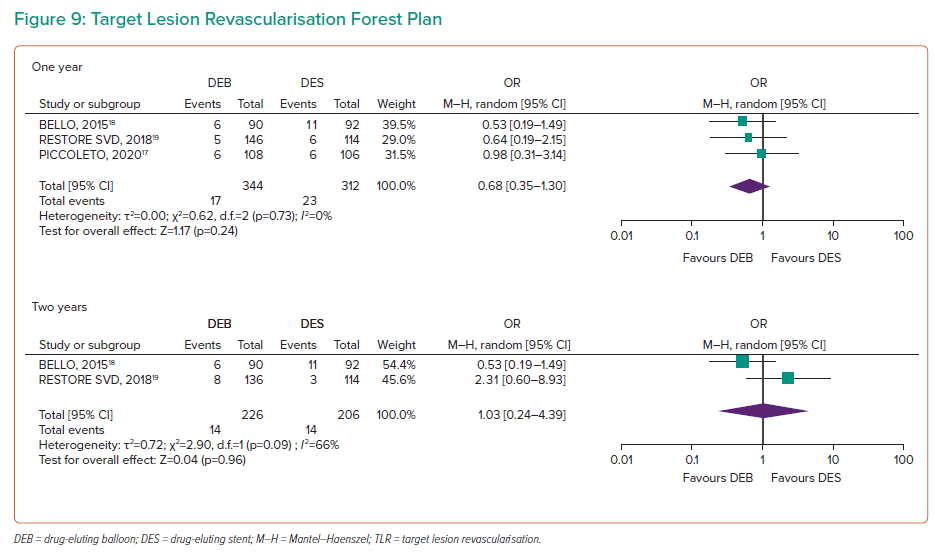
Target Lesion Revisualisation
Target lesion revisualisation (TLR) at 1 year was available for BELLO, RESTORE SVD and PICCOLETO 2 with 615 participants, indicating no significant difference between the two study arms (OR 0.68; 95% CI [0.35–1.30]; Figure 9).17–19 RESTORE SVD and BELLO reported non-significant 2-year outcomes (OR 1.03; 95% CI [0.24–4.39]). RESTORE reported 3-year outcomes of TLR with no significant difference (OR 2.55; 95% CI [0.67–9.65]).18,19 The 1-year data had an I2 of 0%, while 2-year data had an I2 of 66%, above the predetermined threshold for heterogeneity. No change in significance was found in the sensitivity analysis.
Discussion
The present meta-analysis of long-term follow-up RCT data demonstrates DEBs to have comparable performance to DESs in small coronary arteries for MACE, all-cause mortality, cardiac death, MI, vessel thrombosis, major bleeding, TVR and target lesion revascularisation at >1 year of clinical follow-up. Whereas DEBs were superior to DESs regarding MI rates at 1 year (OR 0.44; 95% CI [0.2–0.94]). DESs did not outperform DEBs in any clinical outcome.
Non-randomised trials and registry data comparing DEBs and DESs have shown conflicting results. The SCAAR registry in Sweden included 14,788 patients undergoing percutaneous coronary intervention on small coronary arteries (<2.5 mm between 2009 and 2017) and reported DEBs to have an increased risk of restenosis compared with DESs (adjusted HR 2.027; 95% CI [1.54–2.67]), while there was no difference in all-cause deaths (HR 1.178; 95% CI [0.99–1.4]) or target lesion thrombosis (hazard ratio 0.741; 95% CI [0.41–1.33]).7 Furthermore, plain old balloon angioplasty also demonstrated a trend of increasing lesion restenosis before drug-eluting technology.23 Increased DEB restenosis rates were also present in the first PICCOLETO (2010) trial, which was stopped prematurely due to high rates of MACE at 9 months with the DIOR balloon.24 Importantly, the PICCOLETO 2 (2020) trial included in this meta-analysis used the ELUTAX SV DCB and showed angiographic superiority of DEBs in late lesion loss with no significant increase in the MACE rates.17 The SCAAR registry also included a DEB group with higher coronary disease risk factors and did not routinely perform angiographic follow-up. Additionally, results of our meta-analysis do not indicate a difference between the rates of TVR and TLR at 1, 2 or 3 years of follow-ups in the RCTs, while a non-significant trend favoured DEBs for these outcomes.
It is important to note the potential technical complications of increased use of DEBs. They increase the risk of shear stress and associated dissection, elastic recoil, and abrupt closure.25 The rates of bailout stenting due to coronary artery dissection are 3–20% in the published literature.26 A wide heterogeneity of bailout stenting rates was found among the trials in the included studies. RESTORE SVD reported 5.2%, BELLO 20.2% and the first 2010 PICCOLETO trial 34.5%, while the included second PICCOLETO 2 trial reported a rate of 6.8%. 17–19,24 These differences are hypothesised to be due to technological differences and varying degrees of predilatation pressures, which may affect dissection rates and follow-up TLR rates.27 These data highlight the implications of different operator approaches with DEBs.
The present meta-analysis is supported by two other published meta-analyses: Megaly et al. and Wu et al.28,29 This meta-analysis reports novel follow-up trial data and, to our knowledge, this is the meta-analysis with the longest clinical outcome data reported in the literature. We included the 3-year follow-up of BASKET-SMALL 2 and RESTORE SVD, and 2-year data for BELLO.16,18,19 These data highlight the continued similarity of DEB to DES in the long-term data and further support ongoing safety to the adaption of drug-eluting balloons for SvCAD.
Finally, the included studies present a high-risk group with a high burden of comorbidities. Rates of dyslipidaemia (48–73%), smoking (10–32%), diabetes (32–45%) and previous percutaneous coronary intervention (33–64%) support an ongoing need for appropriate prevention of modifiable risk factors to reduce the burden of small coronary artery disease.30 Of particular concern were the high rates of active smokers among all RCTs, a risk factor with an OR of 2.2 (95% CI [1.28–3.78]) for presenting with an acute coronary syndrome.31
Limitations
Some limitations should be addressed. First, robust randomised control trials on DEBs versus DESs are scarce, and many of the initial trials were excluded for short- to medium-term follow-up. Long-term data of the included trials was often collated from studies after initial publication. Furthermore, with rapidly advancing technology, techniques and medications, the long-term results risk being out of date at publication. This is highlighted by advances in intravascular ultrasound and optical coherence tomography since the RCTs.
Second, the RCTs included in this meta-analysis are primarily aimed at angiographic endpoints rather than long-term clinical outcomes and are weighted to BASKET-SMALL 2.16 Third, each RCT varied in the respective technology and company provider used in each arm; first and second generations of DESs and DEBs were included. Particularly, the differences between the results of the 2010 PICCOLETO 1 and 2020 PICCOLETO 2 indicate differing performances of balloon technologies between studies over a period of 10 years.17,24 Fourth, the literature lacks a clear consensus among researchers on the definition of ‘small coronary arteries’. Definitions in the screened studies ranged from <3 to <2.25 mm with associated heterogeneity of outcomes. Fifth, MACE and TLR outcomes included moderate heterogeneity among the included RCTs.
The composite primary outcome for MACEs remains controversial. No clear definition or consensus exists on what MACEs entail, leading some parties to advise against using this term.32 In the included studies, MACE definitions varied based on the definition of MI and the cause of death recorded. Finally, the included RCTs lacked subgroup analysis of patient cohorts without distinction between outcomes of different comorbidities and patient characteristics.
Conclusion
Long-term follow-up of DEB and DES use in small coronary arteries demonstrates DEBs to be comparable with DESs in all included clinical and angiographic outcomes. Furthermore, DEBs demonstrated significantly reduced rates of non-fatal MI at 1 year, while the BASKET-SMALL 2 trial demonstrated significantly reduced rates of bleeding at 2 years.16 The sustained performance of DEBs over 3 years of follow-up demonstrates a role for DEBs in treating small coronary artery disease.